Oncology
Immuno Oncology
Anti-Virus
Disease Modeling
Cosmetics
Toxicity
Spatial Biology
Technology Service
Normal Organoid
Cancer Organoid
Research Service
Others
Oncology
Immuno Oncology
Anti-Virus
Disease Modeling
Cosmetics
Toxicity
Spatial Biology
Technology Service
Cancer Organoid
Research Service
Others


Organism | Bovine |
Product Type | Tissue |
Tissue | Cornea |
Disease |
Applications
In vitro eye irritation (OECD TG 437)
Eye irritation test is in accordance with the Ministry of Food and Drug Safety guidelines, assesses eye irritation potential using bovine corneas.
In vitro eye irritation test (OECD TG492)
Reconstructed human Cornea-like Epithelium (RhCE) test method for identifying chemicals not requiring classification and labelling for eye irritation or serious eyedamage.
The OECD Test Guideline 437, commonly referred to as the Bovine Corneal Opacity and Permeability (BCOP) test, is an in vitro test method used to classify chemicals according to their potential to cause eye irritation or serious eye damage. This test is particularly significant because it offers an alternative to in vivo testing, which traditionally involved using live animals, and thus supports the principles of the 3Rs (Reduce, Refine, Replace) concerning animal testing.
To determine the eye irritation of a test substance, it is applied tothe cornea of a cow.
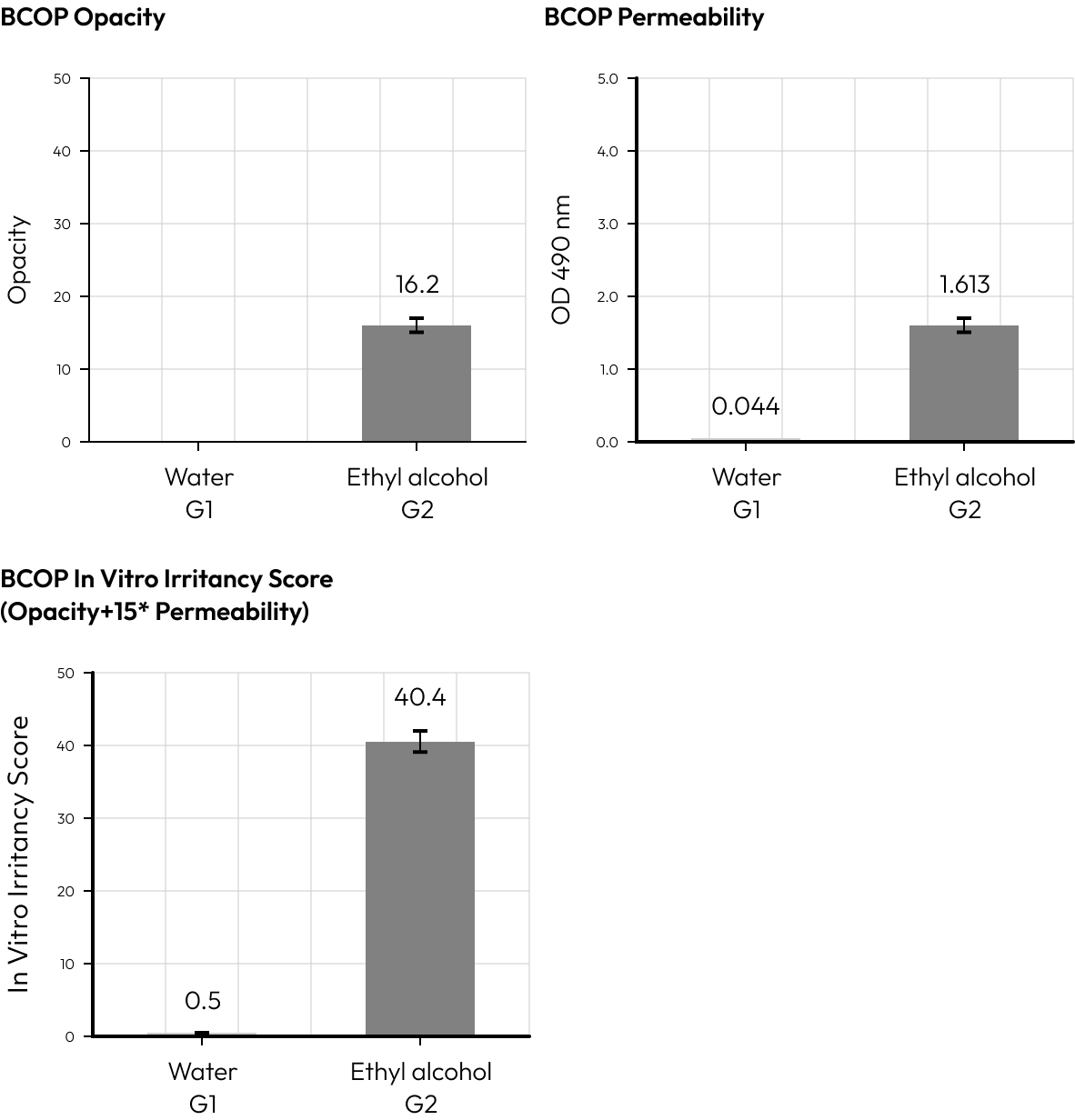
The opacity (cloudiness) and permeability of the cornea are then measured.
These measurements are used to calculate the OcularIrritation Index (IVIS),
which is indicative of the test substance’s eye irritation potential.
Based on the opacity and permeability measurements obtained from the corneal exposure test, the ocular irritation index (IVIS) is calculated.
This index serves as a quantitative measure to evaluate the eye irritation potential of the test substance.
The primary purpose of the BCOP test is to evaluate the hazard of chemicals inducing eye damage through changes observed in both the opacity and the permeability of the bovine cornea, which is a part of the eye that plays a crucial role in focusing vision.
1. Preparation
2. Treatment Application
3. Assessment of Opacity
4. Permeability Measurement
5. Data Analysis
6. Classification

Opacity is assessed by measuring the light transmission through the cornea. This endpoint helps determine the degree of cloudiness or haziness caused by the test item. Permeability Permeability is evaluated by measuring the penetration of fluorescein dye through the cornea. This endpoint indicates the extent to which the test item affects the cornea’s barrier function. Controls Each assay includes both positive and negative controls to ensure the accuracy and reliability of the results. For detailed information on how to test your materials using this assay, please refer to the Applications section. If you require specialized protocols, they can be developed through consultation by My Lab.
MatTek offers the Eye Irritation Test as a GLP or non-GLP service.
@ 2024 . All rights reserved
@ 2024 . All rights reserved