In recent years, the term organoid has gained attention in the fields of biomedical research, drug discovery, and personalized medicine. But what exactly is an organoid? How are organoids created in the lab? And why are they considered revolutionary in modern science?
Let’s explore the journey of these remarkable mini-organs, from their origin in stem cells to their diverse applications and future potential.
What are Organoids?
Organoids are three-dimensional (3D) structures grown in vitro that mimic the architecture and function of real human organs. Derived from stem cells or organ-specific progenitor cells, organoids self-organize into miniature versions of organs such as the brain, liver, lungs, kidneys, and intestines.
What makes organoids special is their ability to recreate key features of the tissue they represent — including cell diversity, tissue structure, and even physiological responses — making them an invaluable model in life science research.
These 3D cell culture models have become a bridge between traditional cell cultures and real human biology, earning their place in the spotlight of translational and precision medicine.
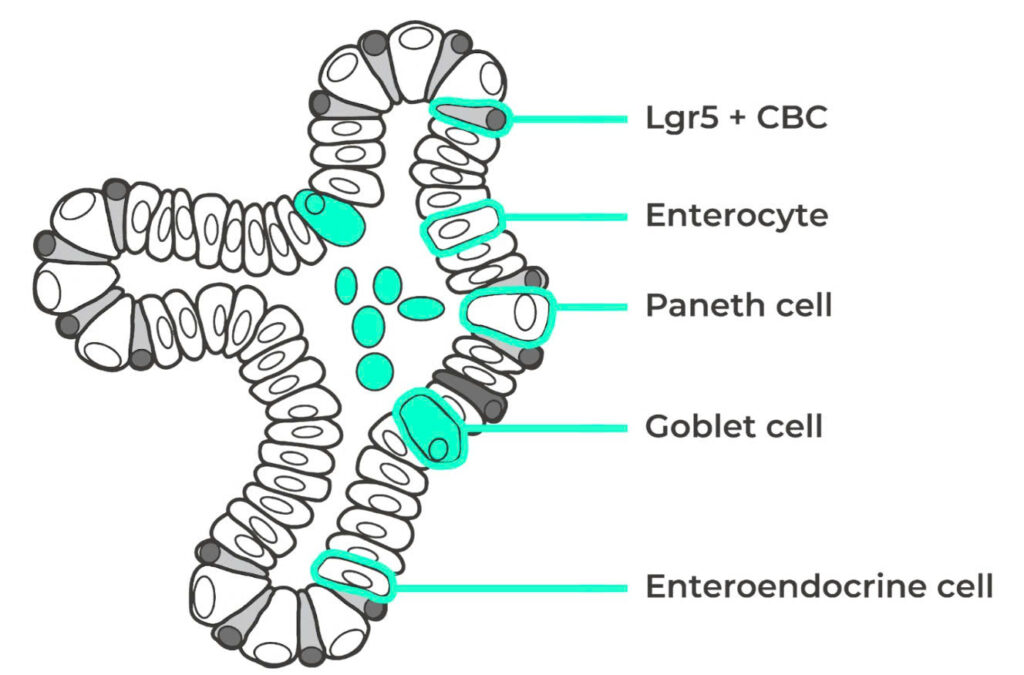
Illustration of intestinal organoid with critical components
How Organoids are Formed in the Lab
The journey of organoid formation begins with stem cells, which have the unique ability to self-renew and differentiate into various cell types. In the lab, scientists culture these cells under specific conditions that mimic the natural environment of a developing organ.
There are two main types of stem cells used in organoid generation:
Pluripotent stem cells (PSCs) – including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), which can differentiate into any cell type.
Adult stem cells (ASCs) – which are tissue-specific and typically found in organs like the intestine or liver.
Through carefully designed 3D culture systems—often using extracellular matrices—these cells are guided to self-organize into organ-specific architectures. The process relies on the cells’ intrinsic developmental programming, combined with external growth factors, to form complex and functional structures.
This technique is often referred to as organoid technology, and it allows for the long-term culture and expansion of mini-organs in dishes.
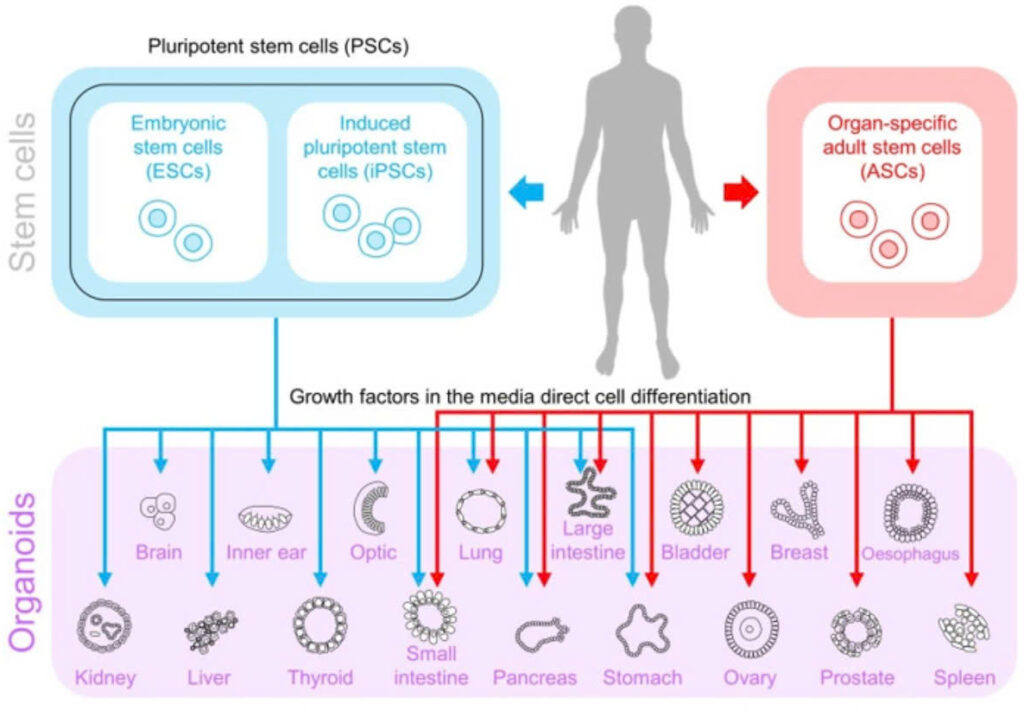
Illustration of organoid formation, from stem cells into different types of “mini-organs”
Platform Comparison: Organoids vs. 2D Cell Culture, Animal Models, and other 3D Models
Organoids represent a significant leap forward in modeling human biology compared to traditional platforms:
2D cell culture: While widely used, 2D cultures lack the spatial organization and cell diversity of real tissues. They often fail to replicate how cells behave in a real organ environment.
Animal models: Animals like mice and rats have been foundational in biomedical research, but key differences in physiology and genetics can limit how well they translate to human biology. Ethical considerations and high costs are additional challenges.
Other 3D models: Spheroids and tissue explants are also used in 3D culture systems. However, spheroids are generally less organized than organoids, and tissue explants are often limited in viability and scalability.
In contrast, organoids offer a human-specific, physiologically relevant, and scalable alternative, combining the complexity of tissue architecture with the ethical and cost advantages of in vitro systems.
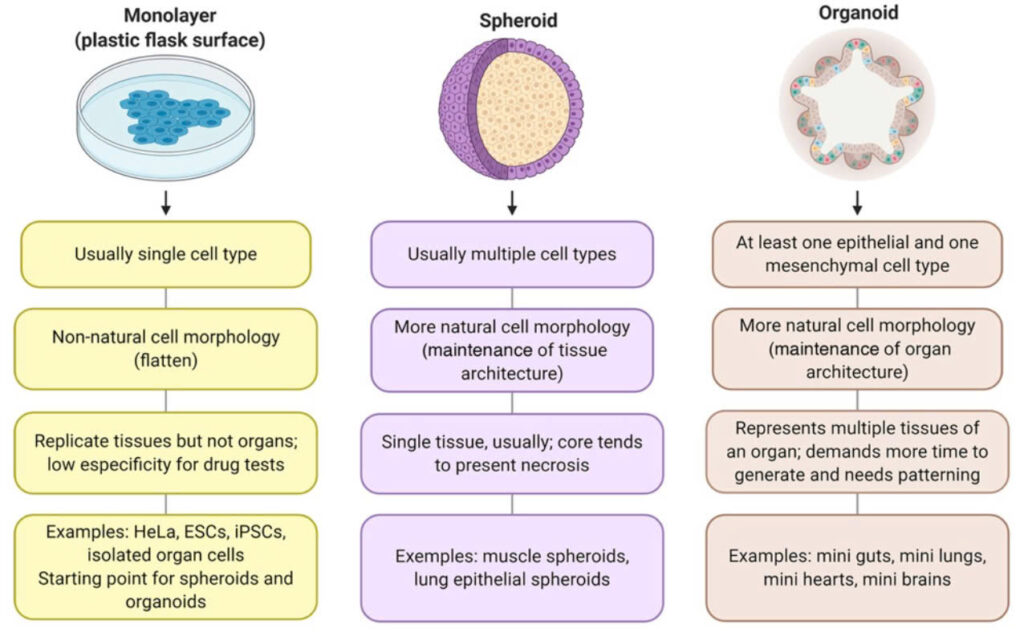
Illustration of different platforms, including 2D cell culture, spheroid, and organoids (left to right)
Applications of Organoids
Organoids are transforming multiple fields of science and medicine. Here’s how:
Biomedical Research
Organoids serve as powerful tools to study development and disease. By mimicking the cellular environment of human organs, scientists can investigate how tissues grow, how diseases like cancer develop, and how genetic mutations influence organ function.
Moreover, scientists can model diseases using organoids. Creating organoids that carry patient-specific genetic mutations or are exposed to disease triggers enables scientists to study the onset and progression of diseases such as cancer, cystic fibrosis, neurodegenerative disorders, and inflammatory bowel disease.
Drug Discovery
Drug screening using traditional 2D cultures often yields incomplete data. Organoids provide a more accurate and human-relevant platform for testing new compounds. Researchers can assess drug absorption, metabolism, and toxicity with greater confidence.
This speeds up the drug discovery pipeline, reduces reliance on animal testing, and increases the likelihood of identifying effective candidates earlier.
Preclinical Drug Testing
Before a new drug enters clinical trials, it must pass rigorous preclinical testing. Organoids are increasingly being integrated into these studies to evaluate efficacy and safety in a system that closely mimics human tissue.
For instance, liver organoids can be used to assess hepatotoxicity, while tumor organoids help determine the effectiveness of anti-cancer therapies.
Personalized Medicine
One of the most promising uses of organoid technology is in personalized or precision medicine. Patient-derived organoids (PDOs) can be grown from individual patients’ tissues or iPSCs, allowing doctors to test different treatments directly on a patient’s mini-organs.
This approach helps identify the most effective therapies with minimal side effects—especially in cancer treatment, where tumor organoids can guide oncologists in selecting targeted therapies.
Regenerative Medicine
While still in early stages, organoids hold promise in regenerative medicine and organ transplantation. In the future, researchers envision using lab-grown organoids to replace damaged tissues or to support organ repair.
Advances in bioprinting and bioengineering may one day allow these mini-organs to grow into transplantable tissues, reducing dependency on organ donors.
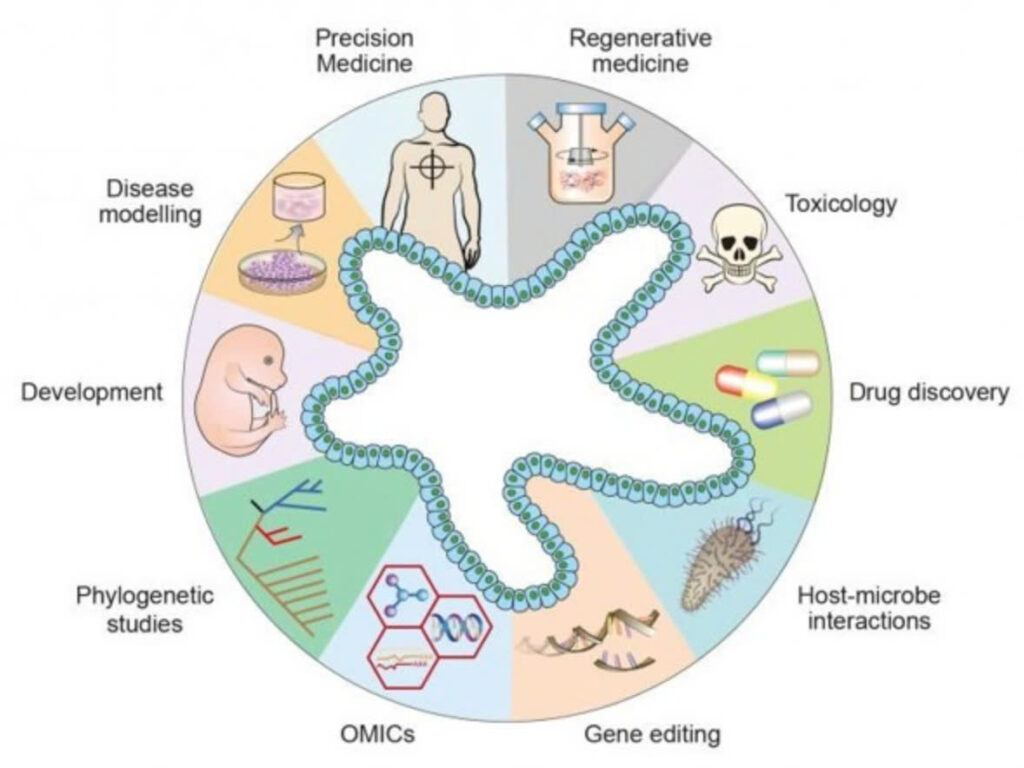
Illustration of organoids in convergence of different fields
Challenges in Organoid Technology
Despite their potential, organoids face several technical and biological challenges:
Vascularization: Organoids often lack blood vessels, limiting their size and maturity. This affects their ability to fully mimic adult organ function.
Reproducibility: Variability between organoid batches can lead to inconsistent results. Standardization of protocols remains a significant hurdle.
Microenvironment complexity: Organoids do not always include immune cells, stromal support, or microbiota, which are critical for modeling disease accurately.
Scale and throughput: While more scalable than animal models, large-scale production of organoids for screening or clinical use remains complex and resource-intensive.
The Future of Organoids
The future of organoid research is incredibly promising. Integration with other cutting-edge technologies—such as CRISPR gene editing, microfluidics, single-cell sequencing, and artificial intelligence—is enhancing our ability to explore complex biological systems.
As these mini-organs continue to evolve, they will undoubtedly play a central role in reshaping biomedical research, reducing animal testing, and bringing precision therapies closer to reality.
Find out more:
Lancaster, M. A., & Knoblich, J. A. (2014). Science, 345(6194), 1247125.
Organogenesis in a dish: Modeling development and disease using organoid technologies.
Clevers, H. (2016). Cell, 165(7), 1586–1597.
Modeling development and disease with organoids.
Yin, X., & Mead, B. E. et al. (2016). Cell Stem Cell, 18(1), 25–38.
Engineering stem cell organoids.
Fatehullah, A., Tan, S. H., & Barker, N. (2016). Nature Cell Biology, 18(3), 246–254.
Organoids as an in vitro model of human development and disease.
van de Wetering, M., Francies, H. E., et al. (2015). Cell, 161(4), 933–945.
Prospective derivation of a living organoid biobank of colorectal cancer patients.
Drost, J., & Clevers, H. (2018). Nature Reviews Cancer, 18(7), 407–418.
Organoids in cancer research.
Kim, J., Koo, B. K., & Knoblich, J. A. (2020). Nature Reviews Molecular Cell Biology, 21(10), 571–584.
Human organoids: model systems for human biology and medicine.
European Molecular Biology Laboratory (EMBL-EBI).
What are organoids?
National Institutes of Health (NIH) News Release (2020).
Organoids as models for drug testing.
National Institutes of Health (NIH) News Release (2020).
Organoids offer alternatives to animal models.
Lambda Biologics’ Oncology Solutions: Patient-derived cancer organoid-based drug evaluation service
Gastric Cancer Organoid | Breast Cancer Organoid | Hepatocarcinoma Cancer Organoid | Pancreatic Cancer Organoid





