As personalized, lab-grown organ replicas are increasingly used to test drugs and facilitate regenerative medicine, researchers in South Korea are in a prime position.
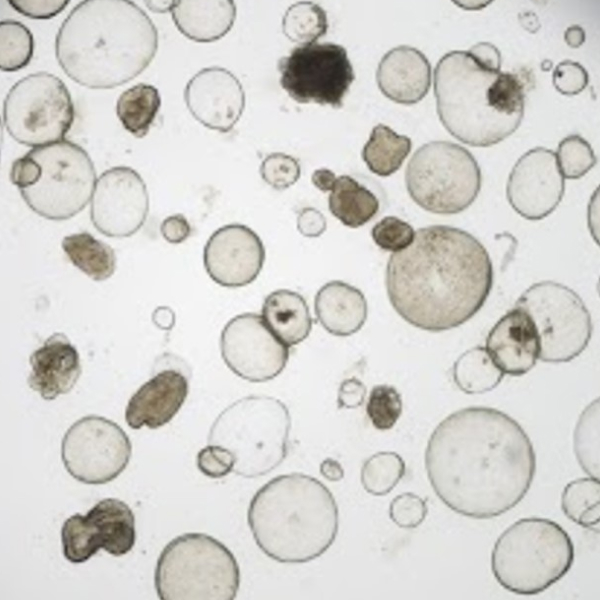
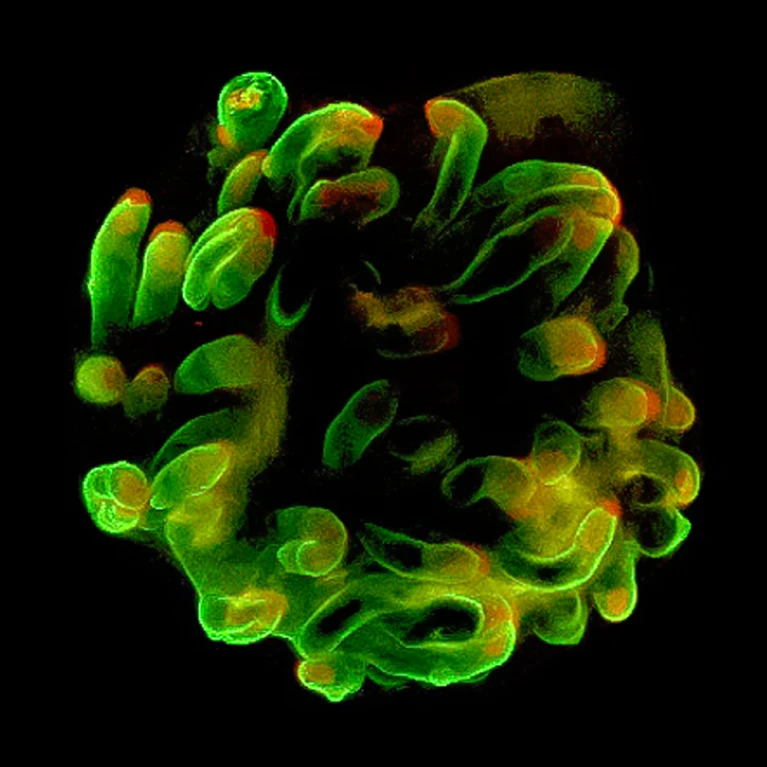
Miniature lab-grown versions of organs, known as organoids, have quickly become key clinical tools — in some cases entirely replacing animal models in pre-clinical safety testing. These three-dimensional, self-organized structures, derived from stem cells, can replicate the architecture of body tissues more faithfully than traditional 2D cultures.
In April 2025, the United States Food and Drug Administration (FDA) announced plans to phase out mandatory animal testing for monoclonal antibodies and other drugs when validated alternatives are available — with cell-based and organoid models now taking a central role in generating reliable safety data for preclinical evaluation. This shift underscores the growing significance of organoid technology in shaping ethical, precise, and innovative biomedical research. It also indicates the technology’s potential to revolutionize regenerative medicine, enable alternative testing, and support precision medicine through patient-derived models.
REGENERATIVE MEDICINE
In regenerative medicine, organoids could increasingly be used to repair, replace, or regenerate damaged or dysfunctional tissues and organs, meaning the research into scaling up their production has become increasingly important. One company hoping to spearhead this is OrganoidSciences, based in Seongnam, South Korea. “We’re the first company in the country dedicated solely to organoid-based regenerative medicine and drug testing,” says CEO Jongman Yoo, who founded the biotech firm in 2018. Since then, OrganoidSciences has developed advanced production methods aimed at scaling up clinical-grade organoid manufacturing, with the goal of producing high concentrations of tissue-specific stem cells that could be used for regenerative purposes.
The company hopes this could be the basis for a fundamental treatment approach that eliminates risks associated with animal-derived materials, such as inconsistent quality and immune reaction problems, while preserving the functional integrity of cultivated organoids^1. They have also been exploring a therapy for refractory inflammatory bowel disease using intestinal tissue organoids, which has recently progressed to human trials.
OrganoidSciences has been recognized by the South Korean Ministry of Trade, Industry and Energy as a company possessing ‘National Advanced Strategic Technology’, which is a designation given to companies that develop technologies with significant national economic benefits and securities. The company is also collaborating with the country’s Ministry of Food and Drug Safety (MFDS) to develop guidelines for organoid-based regenerative therapies.
BEYOND ANIMAL TESTING
Yoo believes that “organoid-driven drug testing is also set to become a gold standard for non-animal drug testing.” Traditionally, drug testing has relied heavily on animal models, he explains — an approach that is increasingly being questioned due to both ethical concerns and the poor translation of results to human biology. Organoid-based testing offers an alternative.
In the field of oncology, organoid-based platforms are rapidly emerging as an alternative to conventional preclinical models. OrganoidSciences has developed tumour organoid models that recapitulate the tumour microenvironment, incorporating stromal and immune components to better reflect patient-specific biology^2. These models enable high-resolution screening of anticancer agents, including immune checkpoint inhibitors, targeted therapies, and combination regimens. By co-culturing tumour-derived organoids with autologous and allogenous immune cells, the models allow for functional prediction of drug responses, providing system for optimizing personalized cancer therapies. Yoo says this approach supports the growing need for individualized medicine, offering a tool to reduce dependency on animal models while enhancing translational relevance.
Alongside its work in therapy development and drug testing, OrganoidSciences is actively involved in establishing standardized organoid methodologies across autoimmune disease, virus, and drug absorption studies^3,4,5. As part of the MFDS-led initiative, it contributes technical expertise to help shape global best practices.
GLOBAL PROPOSITION
Expanding globally through its German strategic partner, Lambda Biologics GmbH, OrganoidSciences seeks to become a central hub for innovative regenerative therapies and advanced animal-free testing platforms. Backed by public funding from the European Union and the government of the Free State of Saxony in Germany, Yoo says that Lambda Biologics serves as a global connector in the shift toward scientifically validated alternatives to animal testing. By leveraging its in vitro testing expertise and platform, Lambda Biologics bridges researchers and solution providers to deliver “innovative, precision-driven testing solutions across the pharmaceutical, cosmetic, and nutraceutical industries.”
André Gerth, CEO of Lambda Biologics, based in Leipzig, Germany, views this global partnership as critical. “With over 20 years of experience in stem cell and regenerative medicine research, I firmly believe organoid-based therapies offer a direct treatment approach distinct from traditional cell therapies,” he says. “Our goal is to integrate global alternative testing methods into a single, intuitive, and efficient platform.”

The ultimate ambition of OrganoidSciences is to develop entire artificial organs. Though this may still be a long-term goal, it clearly defines their vision. Yoo argues that “organoid-based regenerative therapy will eventually underpin the development of artificial organs and extend into everyday applications, from cosmetics and health supplements to precision medicine and pet care.” With growing regulatory support and increasing global investment, he believes that OrganoidSciences is well positioned to lead the transformation of biomedical research and patient care worldwide.
References
1) Jee, J., Park, J., Im, J. H., Kim, M. S., Park, E., Lim, T., Choi, W. H., Kim, J. H., Kim, W. R., Ko, J. S., Jeong, S. Y., Ko, S. Y., Lee, J. I., Lee, K. J., Jeon, H., Seo, J., Hwang, D., Shin, H. S., & Yoo, J. (2021). Functional recovery by colon organoid transplantation in a mouse model of radiation proctitis. Biomaterials, 275, 120925. https://doi.org/10.1016/j.biomaterials.2021.120925
2) Go, Y., Choi, W. H., Bae, W. J., Jung, S., Cho, C., Lee, S. A., Park, J. S., Ahn, J. M., Kim, S. W., Lee, K. J., Lee, D., & Yoo, J. (2022). Modeling Pancreatic Cancer with Patient-Derived Organoids Integrating Cancer-Associated Fibroblasts. Cancers, 14(9), 2077. https://doi.org/10.3390/cancers14092077
3) Jeong, S. Y., Choi, W. H., Jeon, S. G., Lee, S., Park, J., Park, M., Lee, H., Lew, H., & Yoo, J. (2021). Establishment of functional epithelial organoids from human lacrimal glands. Stem Cell Research & Therapy, 12(1). https://doi.org/10.1186/s13287-021-02133-y
4) Kim, H. K., Kim, H., Lee, M. K., Choi, W. H., Jang, Y., Shin, J. S., Park, J., Bae, D. H., Hyun, S., Kim, K. H., Han, H. W., Lim, B., Choi, G., Kim, M., Lim, Y. C., & Yoo, J. (2022). Generation of human tonsil epithelial organoids as an ex vivo model for SARS-CoV-2 infection. Biomaterials, 283, 121460. https://doi.org/10.1016/j.biomaterials.2022.121460
5) Kwon, O., Jung, K. B., Lee, K., Son, Y. S., Lee, H., Kim, J., Kim, K., Lee, S., Song, Y., Jung, J., Park, K., Kim, D., Son, M. J., Lee, M., Han, T., Cho, H., Oh, S. J., Chung, H., Kim, S., . . . Son, M. (2021). The development of a functional human small intestinal epithelium model for drug absorption. Science Advances, 7(23). https://doi.org/10.1126/sciadv.abh1586
—
The texts contents of this article ” How organoids could shape biomedical research” were originally produced in partnership with Nature Custom Media as an advertisement feature and published on Nature.com* on 15 May 2025.
*Nature.com is the online version of Nature, a weekly international journal publishing the finest peer-reviewed research in science and technology.
The advertisement feature can be found here: https://www.nature.com/articles/d42473-025-00067-0