Price | 1784€+ Login to see price |
Organism | Human |
Product Type | Tissue derived organoid |
Tissue | Intestine (Colon) |
Disease | Colorectal cancer |
Applications
Toxicity
Small molecules
mRNA
Antibody
Cancer vaccine
Immune Cells
Immuno Oncology with TME
Cytotoxic T cell
TIL (Tumor infiltrating lymphocytes)
Regulatory T cell
Macrophage
CAF (Cancer associate fibroblast)

With the global rise of K-beauty, the cosmetics industry continues to grow steadily. Since the ban on animal testing for cosmetics in Korea in 2017, various alternative testing methods have...

Traditional microscopy methods often require fluorescent labeling to analyze cellular structures, which can be time-consuming and invasive. In contrast, our HT-X1 system allows for high-resolution visualization of cellular morphology without...

Traditional protein analysis has primarily focused on quantifying expression levels within tissue samples. However, recent advances in spatial analysis techniques have shifted attention toward evaluating not only expression levels, but...
Among the many fermented foods we consume, kimchi is particularly known for containing a diverse range of lactic acid bacteria, which are believed to influence the activation of immune cells...
We conducted a study focused on identifying disease-related markers using patient-derived tissue samples. However, traditional methods limited our ability to analyze multiple candidate markers simultaneously, and the limited availability of...
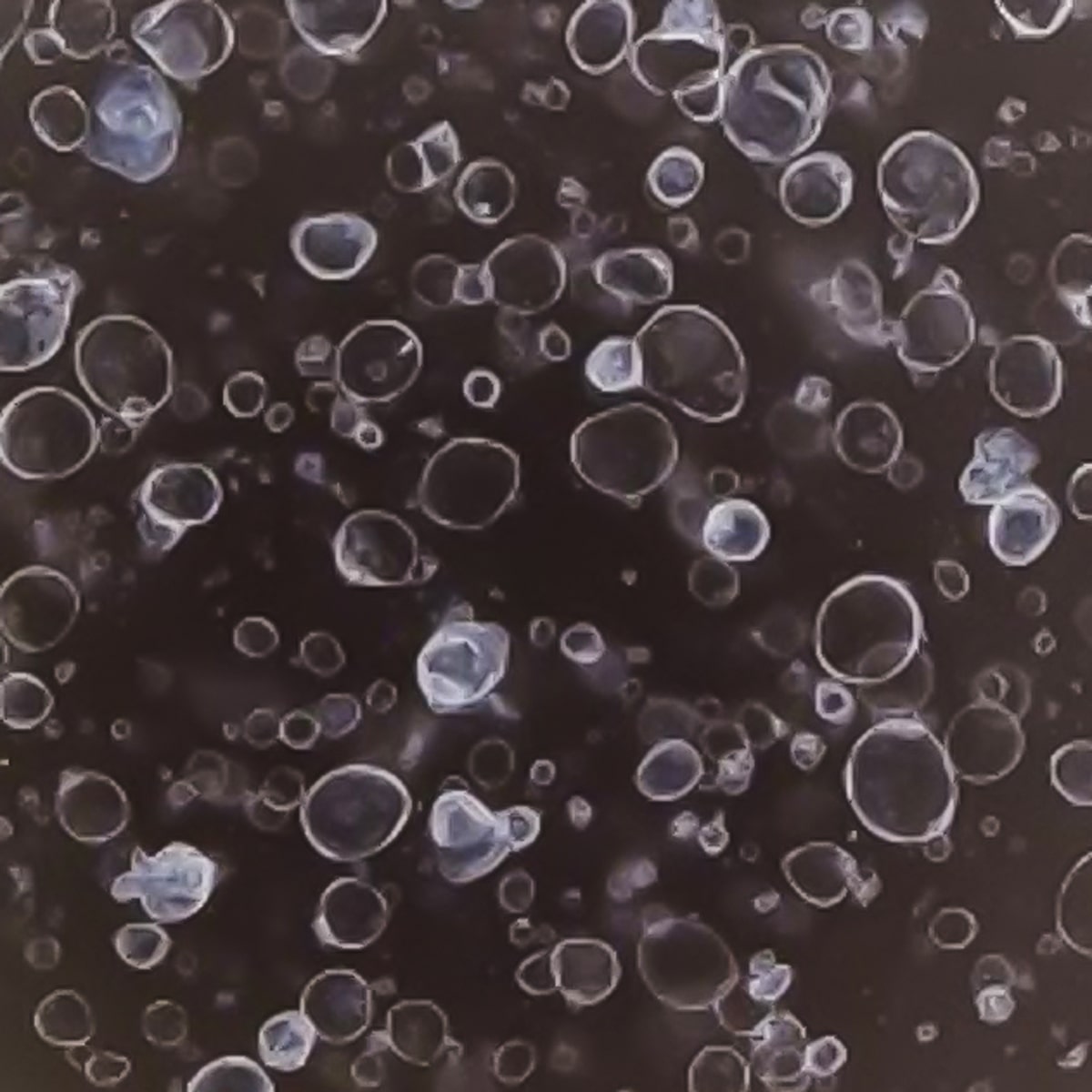
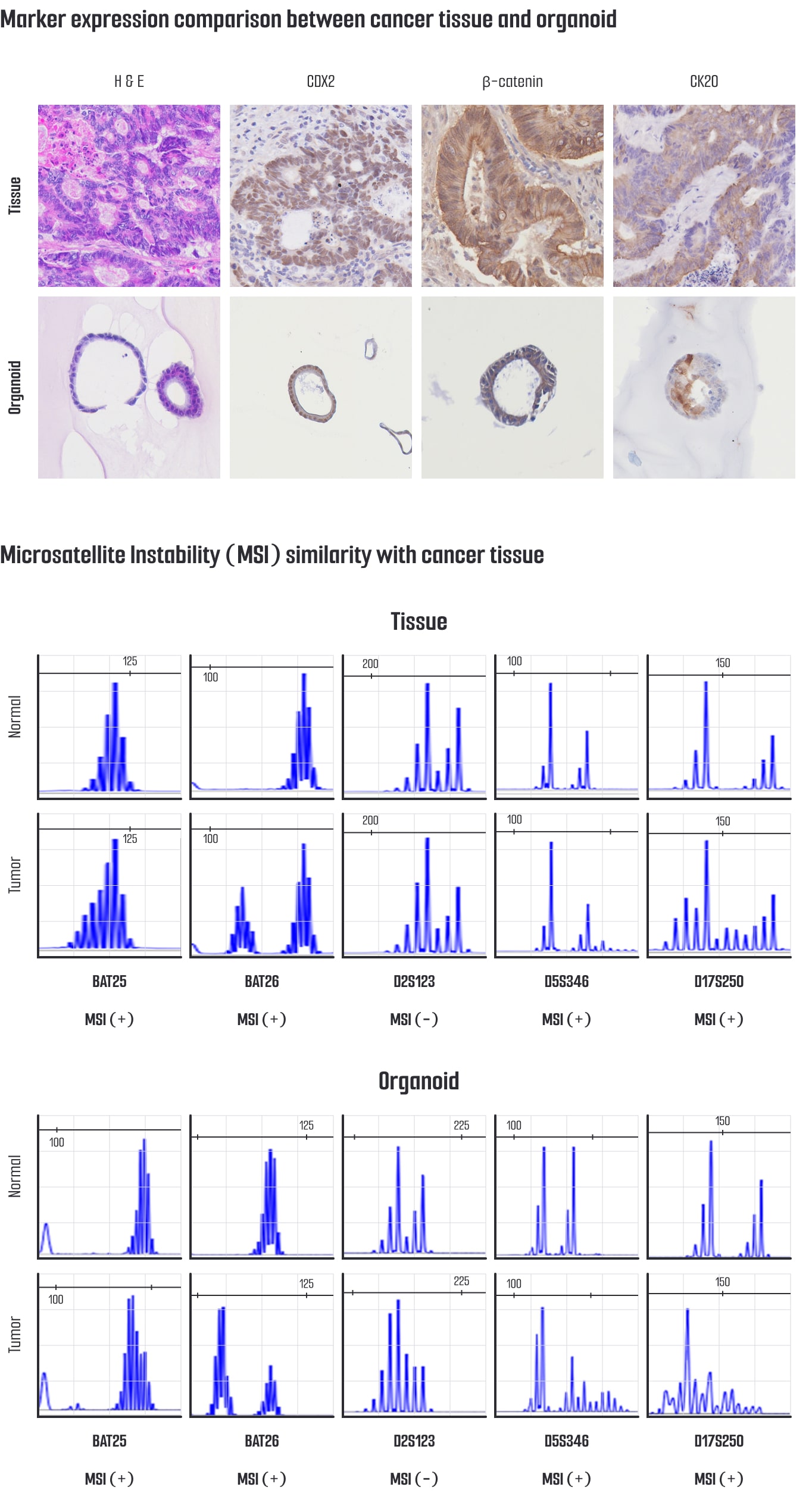
Our colorectal cancer (CRC) organoids exhibit a high degree of pathological similarity to patient-derived tumor tissues, expressing key lung cancer markers such as CDX2, β-catenin (CTNNB1), and CK20 (KRT20).
Immunohistochemistry (IHC) analyses confirm that these markers are expressed in the organoids in patterns consistent with those observed in primary tumors, accurately reflecting the differentiation status and histological characteristics of the original tissue.
These pathological marker analyses demonstrate that CRC organoids faithfully recapitulate the morphological and molecular features of patient tumors, establishing them as a reliable platform for cancer pathology research, drug development, and the evaluation of personalized therapeutic strategies.
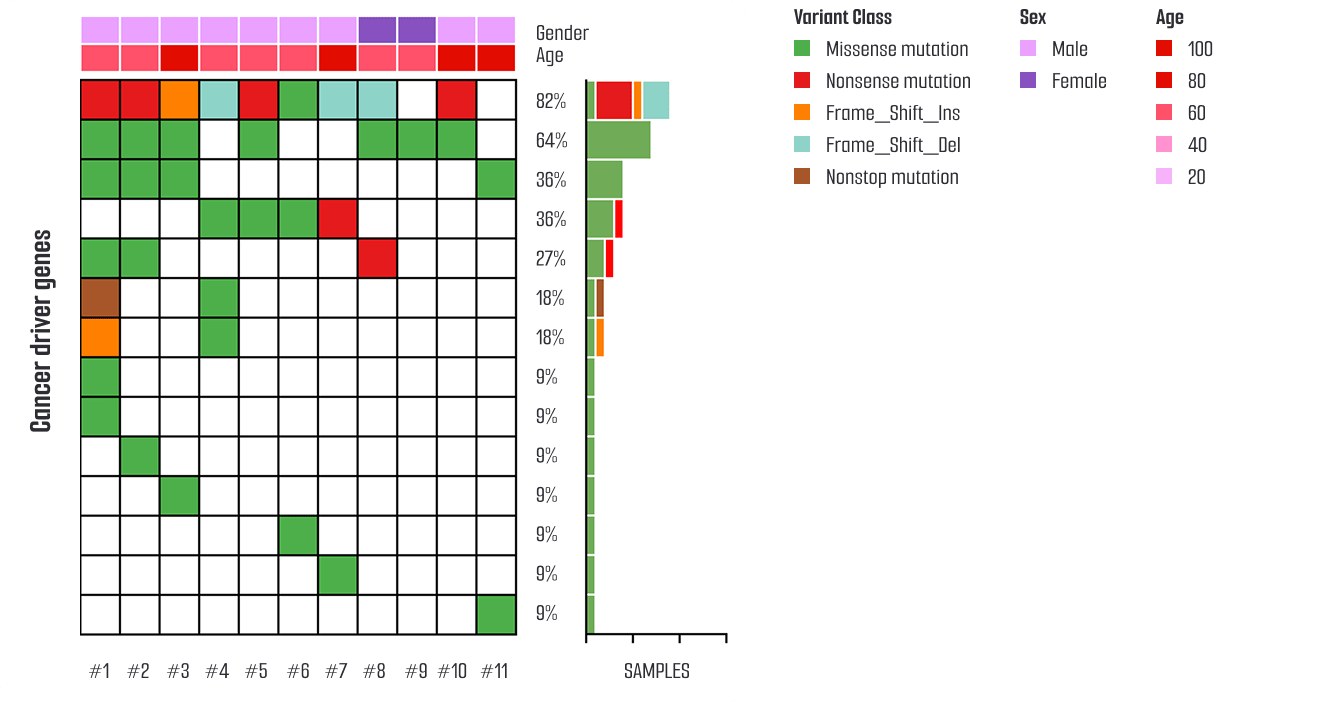
The colorectal cancer (CRC) organoid platform provides a physiologically relevant model that reflects the genetic characteristics of patient tumors.
Whole Exome Sequencing (WES) has been performed on the organoid library, identifying key driver mutations commonly found in CRC.
These genomic profiles are visualized using Oncoplots, providing clear interpretation of the genetic features of each organoid model.
With WES data already established, cancer organoids harboring specific gene mutations can be selectively utilized for targeted drug testing, mechanistic studies, and mutation-driven drug response analysis.
These organoids serve as versatile models that reflect diverse tumor characteristics such as metastasis, treatment resistance, and drug sensitivity, making them effective for evaluating both standard therapies and novel anticancer strategies.
This platform supports mutation-specific organoid selection and testing, positioning it as a powerful tool for precision oncology and the development of personalized cancer therapies.
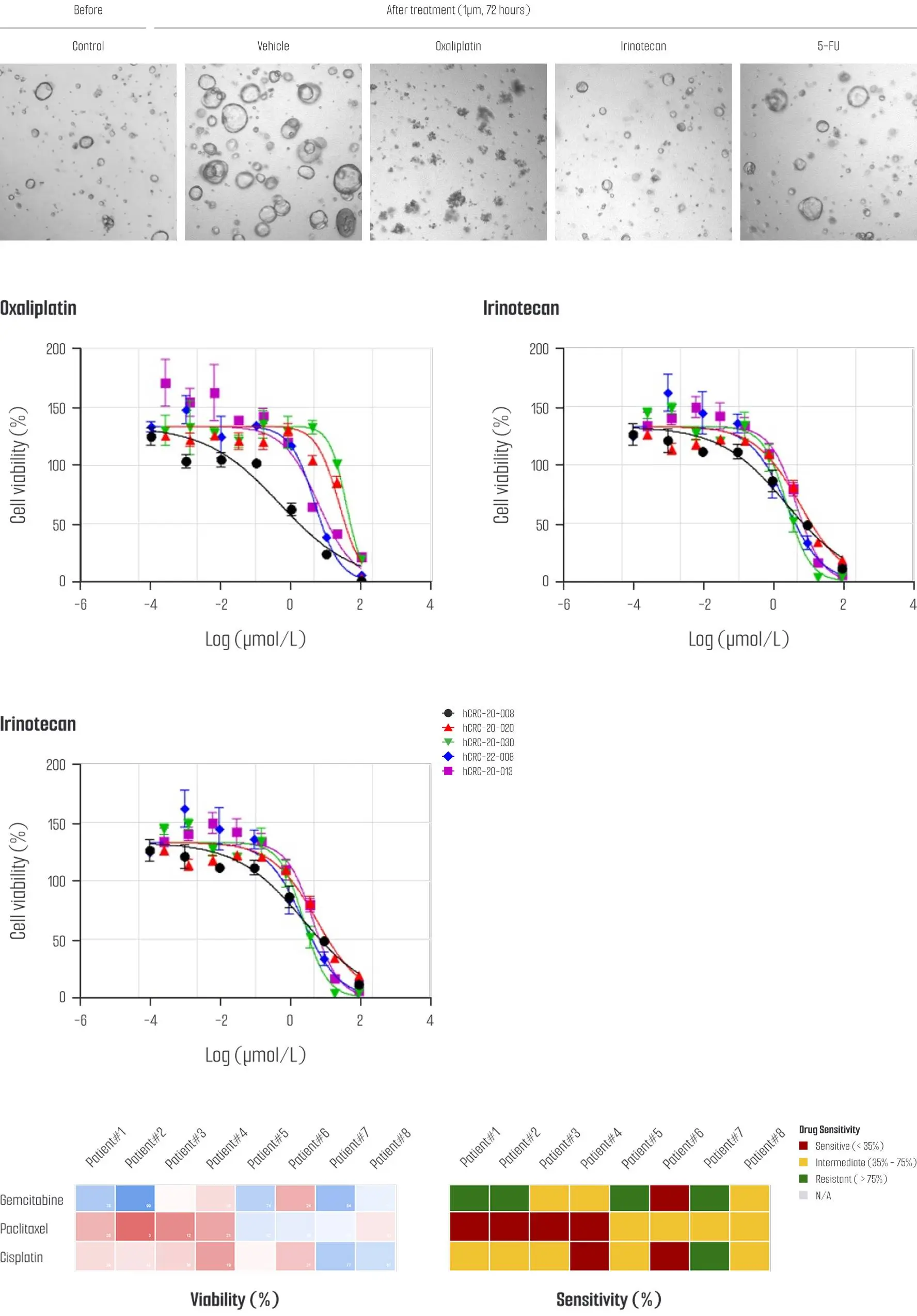
A tailored solution for evaluating the efficacy and resistance of new drugs is available using an established colorectal cancer (CRC) organoid library.
By leveraging comprehensive drug sensitivity data, the most suitable organoid lines are selected based on the characteristics of the investigational drug, enabling precise and reliable drug testing.
Drug sensitivity tests have been conducted using 5-FU, Oxaliplatin, and Irinotecan which are widely used in clinical cancer treatment, and drug response data has been collected from a subset of CRC organoid models, comprising over 20 distinct lines.
This dataset continues to expand as additional testing is performed.
This organoid-based drug sensitivity testing platform enhances the efficiency and reliability of the drug development process and contributes to the advancement of personalized cancer treatment strategies.
Deutscher Platz 5c, 04103, Leipzig, Germany
info@lambdabiologics.com