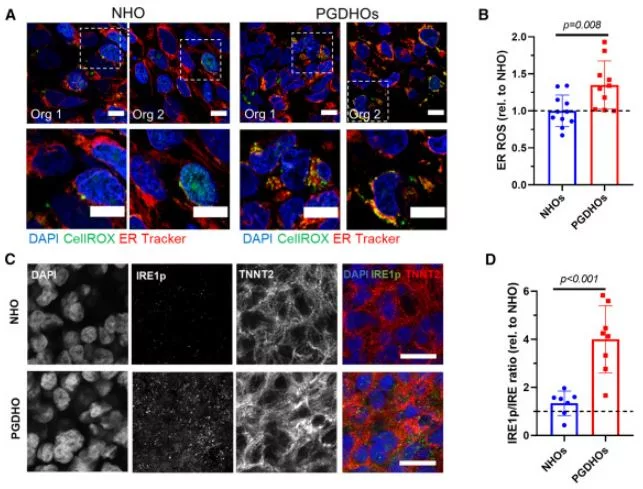
The prevalence of congenital heart defects, a common birth defect, is exacerbated by maternal health conditions like pregestational diabetes during the first trimester of pregnancy. However, our understanding of these disorders is limited due to a lack of human models and accessibility to embryonic tissue. To address this, advanced human heart organoids were used to simulate embryonic heart development under conditions similar to pregestational diabetes. These organoids exhibited pathophysiological features observed in both mouse and human studies, including oxidative stress and cardiomyocyte hypertrophy. Single-cell RNA sequencing (scRNA-seq) analysis revealed specific dysfunction in cardiac cell types, affecting epicardial and cardiomyocyte populations, as well as alterations in endoplasmic reticulum and lipid metabolism. Further imaging and lipidomics studies confirmed these findings, indicating a link between dyslipidemia and fatty acid desaturase 2 mRNA decay mediated by IRE1-RIDD signaling. Targeting IRE1 or restoring lipid levels partially reversed the effects of pregestational diabetes, suggesting potential preventive and therapeutic strategies in humans.
Keywords: Organoids, oncology