Systematic Production of Human Kidney Organoids for Transplantation
Journal: Nature Biomedical Engineering
Author: Garreta, E., Moya-Rull, D., Centeno, A. et al., Spain
A new study has demonstrated a scalable and reproducible method to generate human kidney organoids from pluripotent stem cells – paving the way for organoid-based regenerative therapies. Researchers developed a cost-effective system that ensures consistent differentiation into diverse renal cell types. Using single-cell sequencing, imaging, and metabolic profiling, they confirmed the organoids’ structural and functional similarity to human kidney tissue. Remarkably, these organoids were infused into porcine kidneys via ex vivo normothermic machine perfusion and later shown to successfully engraft in vivo. The findings validate the feasibility of using human kidney organoids in cell-based transplantation and offer a critical step toward clinical applications in organ repair and replacement.
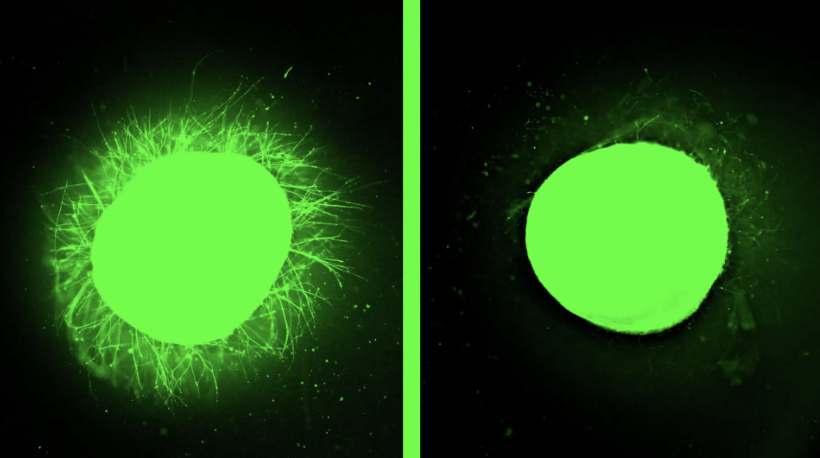
Clonidine Protects Human Brain Organoids from Radiation Damage
Journal: Scientific Reports
Author: Lundberg, M., Samudyata, Koskuvi, M. et al., Sweden
Researchers have discovered that clonidine, a commonly used antihypertensive and sedative drug, can help prevent radiation-induced brain damage – a major challenge in pediatric cancer treatment. Using human brain organoids that model early neural development, the study found that clonidine significantly reduced radiation-triggered cell death across neurons, neural progenitors, astrocytes, and oligodendrocyte lineage cells. It also lessened DNA damage and reactive gliosis, key indicators of neurotoxicity. These results mark the first demonstration that pharmacological protection against radiation injury is possible in a human brain organoid model, offering a promising avenue for drug repurposing to protect young patients undergoing radiotherapy.
New Strategy Targets Cancer’s Glutamine Addiction
Journal: Nature Communications
Author: Sung, Y., Yu, Y.C., Lee, M. et al., Republic of Korea
A groundbreaking study has identified iMQT_020, the first selective inhibitor of the mitochondrial glutamine transporter SLC1A5_var – a key player in fueling cancer cell metabolism. By blocking this transporter, iMQT_020 disrupts the import of glutamine into mitochondria, leading to a metabolic collapse in cancer cells while sparing healthy ones. The compound works allosterically, destabilizing SLC1A5_var’s trimeric structure and halting glutamine-driven energy production and redox balance. Beyond metabolic disruption, iMQT_020 also enhances immune response by epigenetically increasing PD-L1 expression, showing strong synergy with anti-PD-L1 immunotherapy. These findings reveal SLC1A5_var as a critical metabolic vulnerability in cancer and position allosteric inhibition as a promising new direction for developing next-generation anticancer therapies.
AI-Powered Spatial Phenomics Transforms Lung Cancer Risk Prediction
Journal: Nature Communications
Author: Schallenberg, S., Dernbach, G., Ruane, S. et al., Germany
Researchers have developed an AI-driven spatial cell phenomics approach that dramatically improves how clinicians assess risk in non-small cell lung cancer (NSCLC). By integrating histology, multiplex immunofluorescence imaging, and multimodal machine learning, the model analyzed cellular interactions among 43 distinct cell types within the tumor microenvironment across more than 1,100 patient samples. The AI system enhanced risk stratification accuracy by 14% in lung adenocarcinoma and 47% in squamous cell carcinoma, uncovering complex immune cell “niches” linked to patient survival. These insights can identify high-risk patients who may benefit from additional therapies. The study demonstrates how AI-powered spatial biology can refine cancer diagnosis, improve treatment decisions, and deepen understanding of tumor–immune dynamics.