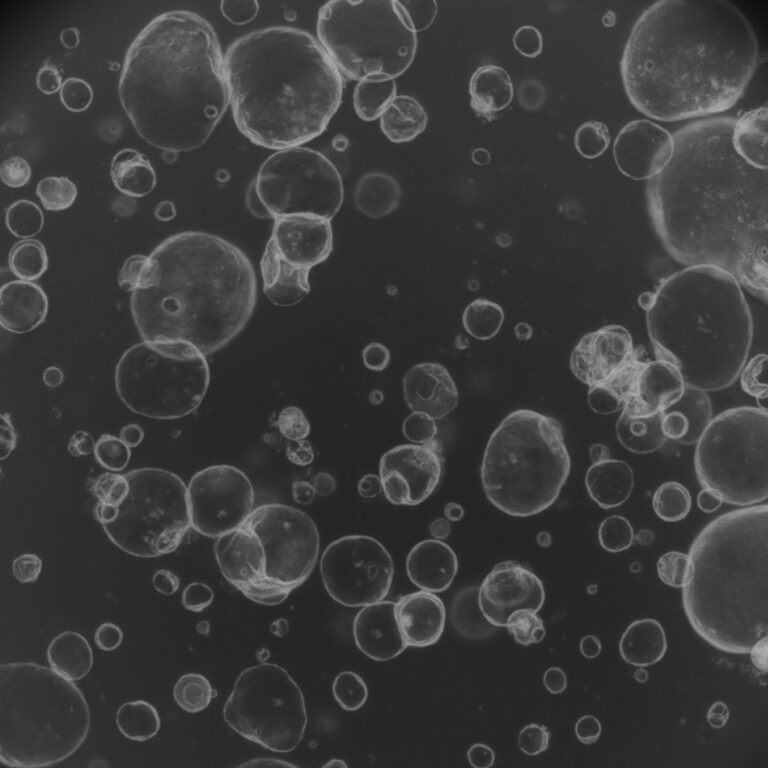
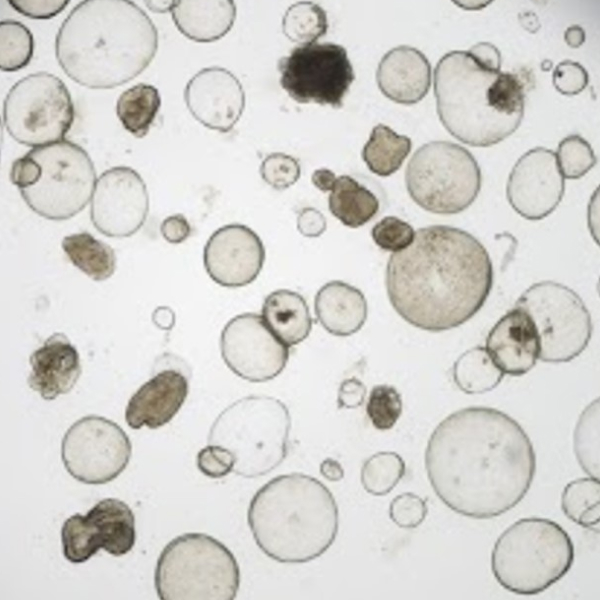
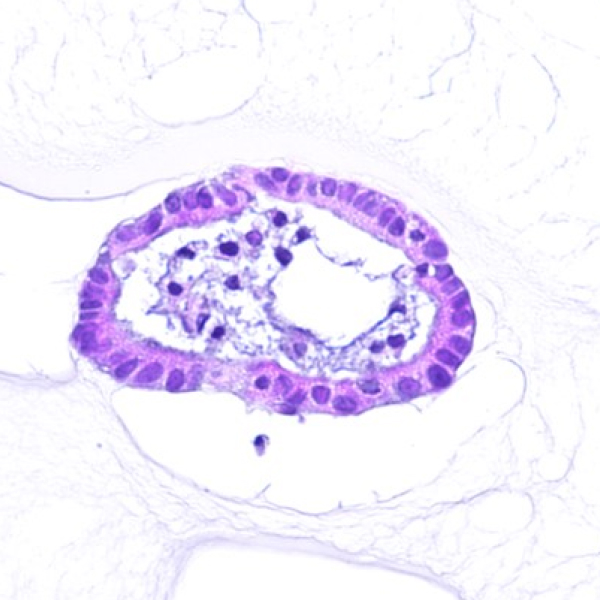
Organoids are three-dimensional (3D) miniature versions of organs grown in vitro that closely mimic the structure and function of real human tissues. These advanced biological models are transforming biomedical research, enabling more accurate studies of disease mechanisms, drug screening, toxicology, and personalized medicine.
Read more: What are Organoids? | From Stem Cells to Mini-Organs
In this blog post, we’ll walk you through the essential steps, materials, and media needed to grow organoids in the lab – from selecting the appropriate cell sources to maintaining long-term cultures and preserving organoids for future use.
Choosing the Right Stem Cells for Organoids
Organoids are derived from two main types of stem cells, depending on the scientific objective. Pluripotent stem cells (PSCs), such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have the ability to differentiate into any cell type in the body. This makes them ideal for modeling early stages of organ development, also known as organogenesis.
Adult stem cells (ASCs), also called multipotent stem cells, are more limited in their differentiation potential but retain the features of the tissue they are isolated from. ASCs can be used to generate patient-derived organoids (PDOs), and are particularly useful for disease modeling and regenerative medicine applications.
Generating Organoids from Tissue or Cell Lines
Organoid generation begins by isolating and preparing cells for 3D culture. Here’s a simplified protocol using tumor tissues (e.g., colorectal cancer):
Tissue dissociation: Use mechanical and enzymatic methods (e.g., collagenase) to break down tissue into a single-cell suspension.
Filtration and lysis: Filter to remove debris and lyse red blood cells or unwanted components.
Cell sorting: Enrich specific populations (e.g., CD133+ cancer stem cells) using FACS or MACS.
Spheroid formation: Culture sorted cells in low-attachment flasks to form tumor spheroids.
Media monitoring: Change media every 2–3 days and monitor pH and cell health daily.
Passaging: After ~7 days, dissociate spheroids and embed single cells into a hydrogel-based matrix (usually Matrigel or BME).
Organoid culture: Seed cells into dome-like structures in 3D matrix, enabling maturation into complex organoids.
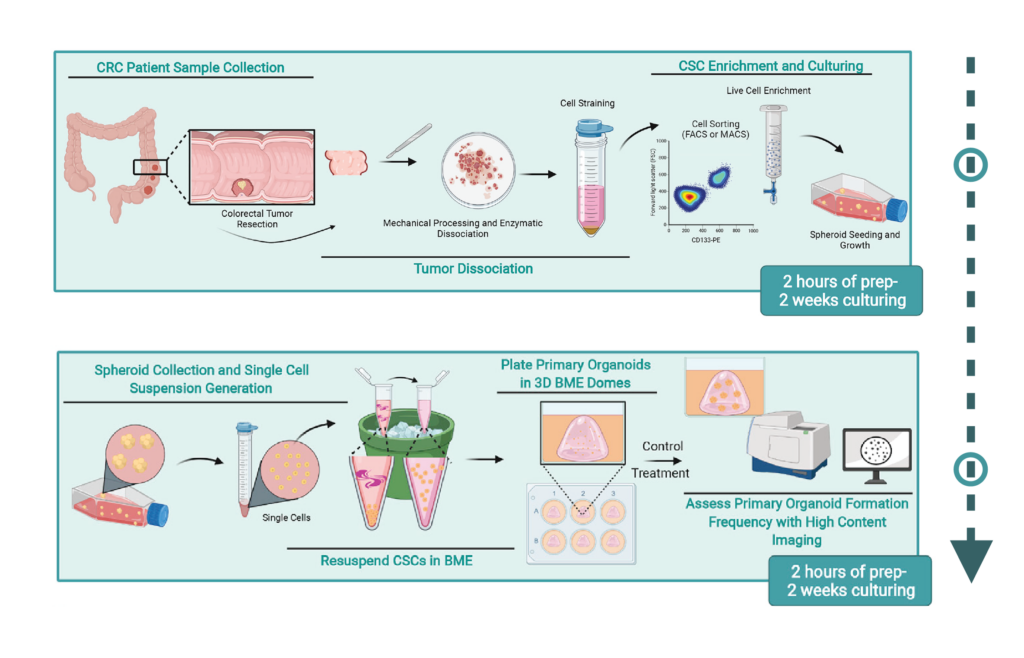
From patient tissue to 3D cancer organoid culture (CSC = cancer stem cells). Image source: Fig.1 and 3 cropped and added arrow (↓): https://doi.org/10.1016/j.xpro.2022.101218
Selecting the Right Matrix and Media
To support the growth and development of organoids, specific signaling molecules are added to the culture media. These help control how cells grow, stay alive, and take on specialized functions.
Key growth factors:
- EGF: Stimulates cell proliferation, important in the growth of cancer and other cell types.
- FGFs: Act as signaling molecule that promotes cell division. Multiple functions on multiple cell types.
- Wnt: Crucial in early development, determining cell fate, growth, and migration.
- R-Spondin: Enhances Wnt signaling via Wnt/β-catenin pathway.
- Noggin: Inhibits BMP signaling and support proper tissue patterning.
- Gastrin: Supports growth.
Small molecule inhibitors:
- A83-01: Blocks TGF-β signaling, preventing unwanted differentiation.
- Nicotinamide: A form of vitamin B3 that improves cell metabolism.
- SB202190: Inhibits stress-related signaling, aiding in stable growth.
- CHIR99021: Enhances Wnt signaling by inhibiting GSK3.
- Y27632 (ROCK inhibitor): Prevents cell death by blocking Rho kinase.
Summary of specific media components for different organoids:
Organoid Type | Base Media | Growth Factors & Supplements |
|---|---|---|
Cerebral (Brain) | Neurobasal + NEAA | Retinoic Acid, R-Spondin, Noggin |
Intestinal | DMEM/F12 + FBS | EGF, R-Spondin, Noggin, Wnt |
Liver | DMEM/F12 + FBS | EGF, R-Spondin, FGF, HGF, Nicotinamide, Insulin |
Pancreas | DMEM/F12 + B27 | FGF, EGF, R-Spondin, Nicotinamide, Noggin, Wnt, A83-01, Gastrin |
Prostate | DMEM/F12 + FBS | EGF, R-Spondin, Noggin, Dihydrotestosterone |
Lung | DMEM/F12 + FBS + B27 + N2 | GlutaMAX, FGF, Noggin, SB431542, CHIR99021 |
Mammary | DMEM/F12 + FBS + ITS | EGF, Hydrocortisone, T3, β-Estradiol, Isoproterenol, Ethanolamine, Phosphoethanolamine |
Ovary & Fallopian | DMEM/F12 + FBS | R-Spondin, FGF, EGF, Noggin, Nicotinamide, Wnt |
Kidney | DMEM High Glucose + FBS + NEAA | GlutaMAX, Heparin, APEL, FGF9, SB431542, CHIR99021 |
Bladder | Hepatocyte Medium + FBS | EGF, GlutaMAX, Primocin |
Esophageal | DMEM/F12 | EGF, Noggin, R-Spondin, Wnt, SB202190, A83-01, Nicotinamide, Gastrin |
The extracellular matrix (ECM) plays a critical role in supporting organoid development by providing structural integrity and biochemical signals. Among the natural ECM options, Matrigel (produced by Corning) is one of the most widely used. It contains key components such as laminin, collagen IV, and growth factors that closely mimic the basement membrane environment.
In contrast, synthetic hydrogels offer customizable stiffness and composition. However, they often require chemical modifications to interact effectively with cells, and their structural properties can sometimes hinder long-term organoid development. As a result, while Matrigel offers excellent biological compatibility, synthetic matrices provide standardization and flexibility for experimental design.
Organoid Culture Techniques
Organoid culture methods vary depending on the type of organ and experimental goal. In the earlier protocol, suspension culture is used to expand spheroids in low-adhesion flasks. Once cells are ready, they are transferred to embedded culture systems where they are seeded in 3D matrices and allowed to grow in domes within culture plates.
For specialized applications, other methods may be used. Air-liquid interface (ALI) systems are ideal for respiratory or gastrointestinal organoids, as they enhance oxygen and gas exchange. For long-term or large-scale organoid cultures, bioreactors provide mechanical stimulation and improved nutrient perfusion, which supports healthier and more mature organoid growth.
Characterizing Organoids for Ensuring Quality and Relevance
Before organoids can be used for experimental studies, they must be thoroughly characterized to ensure that they accurately replicate native tissue features. Brightfield microscopy allows researchers to assess overall morphology. Histology and immunostaining are used to detect tissue-specific markers, which indicate successful differentiation.
Molecular profiling methods – including quantitative PCR (qPCR), RNA sequencing, and whole-genome sequencing – are employed to analyze gene expression patterns and detect mutations. Comparative analysis with the original tissue sample is crucial to confirm that the organoids retain the biological traits of the donor tissue.
Downstream Applications and Organoid Preservation
Organoids can be used in various downstream applications. These include high-throughput drug screening, toxicity testing, disease modeling, and developmental biology research. Organoids are also increasingly used in personalized medicine to evaluate patient-specific responses to treatments.
To preserve organoids for future experiments, a cryopreservation protocol is followed. The organoids are first harvested and dissociated into small clumps. They are then resuspended in a cryoprotective medium containing dimethyl sulfoxide (DMSO). After slow freezing using a controlled-rate cooling device, the samples are stored in liquid nitrogen. Upon thawing, depending on the requirement of specific organoid model, a ROCK inhibitor such as Y-27632 could be added to enhance cell survival and recovery.
Organoid culture is a powerful and ethical alternative to traditional animal models, enabling more human-relevant insights into biology and disease. While protocols may vary depending on the tissue type and research goals, mastering the fundamental materials, media, and methods discussed in this guide will help researchers build robust, reproducible, and impactful organoid systems.
References
Artegiani, B., & Hendriks, D. F. G. (2025).Organoids from pluripotent stem cells and human tissues: When two cultures meet each other. Developmental Cell, 60(4), 493–511. DOI: 10.1016/j.devcel.2025.01.005
ATCC. (n.d.). Organoid culture guide. https://www.atcc.org/resources/culture-guides/organoid-culture-guide
Corning Life Sciences. (n.d.). Matrigel® Matrix. Corning. https://www.corning.com/worldwide/en/products/life-sciences/products/surfaces/matrigel-matrix.html
Rauth, S., et al. (2021). Recent advances in organoid development and applications in disease modeling. Biochimica et Biophysica Acta (BBA) – Reviews on Cancer, 1875(2), 188527. https://doi.org/10.1016/j.bbcan.2021.188527
Bergin, C. J., & Benoit, Y. D. (2022). Protocol for serial organoid formation assay using primary colorectal cancer tissues to evaluate cancer stem cell activity. STAR Protocols, 3(1), Article 101218. https://doi.org/10.1016/j.xpro.2022.101218
News-Medical. (n.d.). Methods of Organoid Cultures. News-Medical. https://www.news-medical.net/life-sciences/Methods-of-Organoid-Cultures.aspx news-medical.net
News-Medical. (2023, December 13). Organoid Models for Cancer Research. News-Medical. https://www.news-medical.net/whitepaper/20231213/Organoid-Models-for-Cancer-research.aspx
Sino Biological. (n.d.-a). Colorectal Cancer Organoids: Definition, Applications, Protocol, and Characterization. Sino Biological. https://www.sinobiological.com/resource/organoid-review/colorectal-cancer-organoids
Sino Biological. (n.d.-b). Organoid Culture: Definition, Sources, Methods, and Protocols. Sino Biological. https://www.sinobiological.com/resource/organoid-review/organoid-culture
Lambda Biologics’ Oncology Solutions: Patient-derived cancer organoid-based drug evaluation service
Gastric Cancer Organoid | Breast Cancer Organoid | Hepatocarcinoma Cancer Organoid | Pancreatic Cancer Organoid