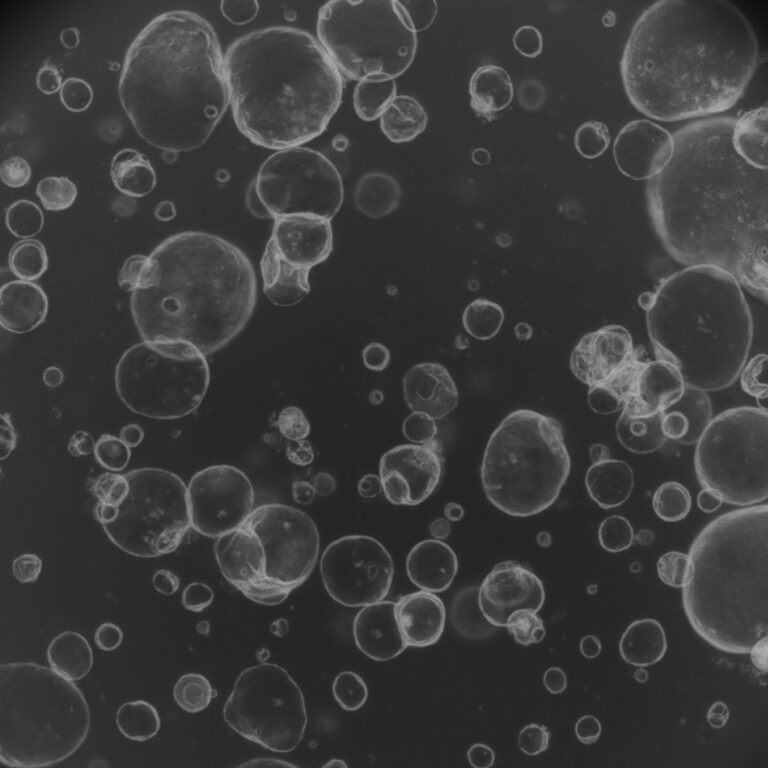
The FDA Modernization Act 2.0, a bipartisan bill, was enacted into law in December 2022. It introduces significant changes to the drug development process by allowing sponsors of novel drugs to use alternatives to animal testing, including organoids, to assess the safety and effectiveness of these drugs. This legislation acknowledges the advancements in non-animal testing methods and offers the option for pharmaceutical and biotech companies to utilize more ethical and scientifically advanced approaches like organoids for drug evaluation.
Keywords: Organoid