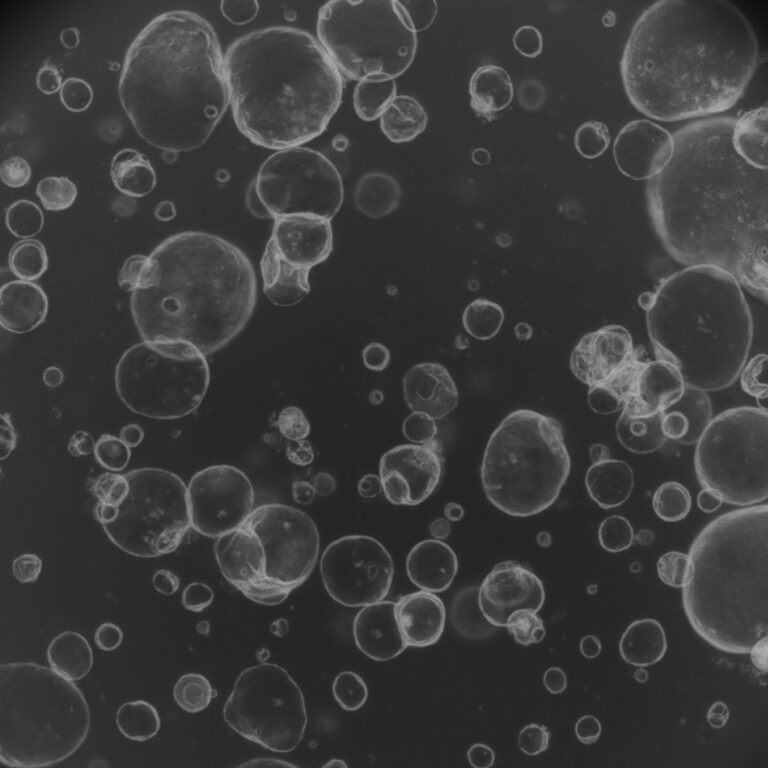
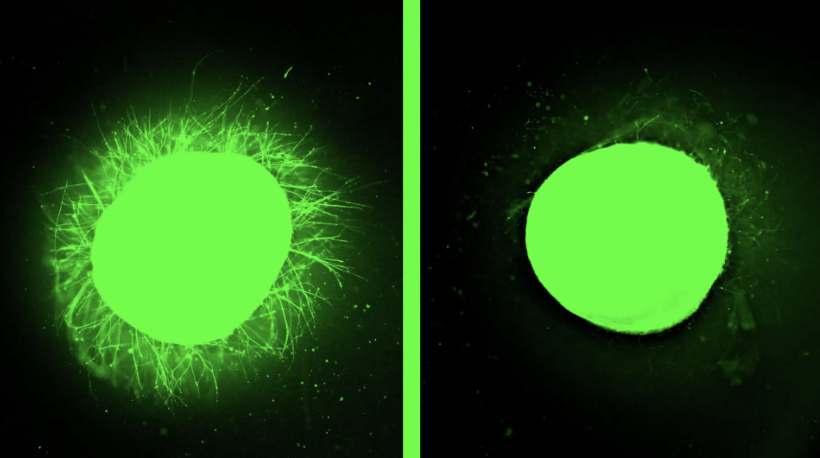
The FDA’s decision to phase out animal testing requirements for monoclonal antibodies signals a transformative moment in medicine. By embracing organoids, AI modeling, and human-derived testing platforms, regulators validate what biotechnology innovators have long championed.
This shift promises accelerated development of treatments while enhancing safety through methods that better reflect human biology. The reduction in animal experimentation aligns with evolving ethical standards and may lower R&D costs.
This landmark decision places human-relevant testing at the center of pharmaceutical advancement—where science and ethics converge.
Meet us at AACR 2026 - April 17-22 - TME Interplay with Organoids
Meet us at AACR 2026 - April 17-22 - TME Interplay with Organoids
Meet us at AACR 2026 - April 17-22 - TME Interplay with Organoids
Meet us at AACR 2026 - April 17-22 - TME Interplay with Organoids
Meet us at AACR 2026 - April 17-22 - TME Interplay with Organoids
Meet us at AACR 2026 - April 17-22 - TME Interplay with Organoids