A co-culturing platform of macrophages and organoids demonstrates varying organoid cytotoxic effects depending on the ratio of M1 macrophages to M2 macrophages.
Optimal cell death effects are established by co-culturing different ratios of functionally diverse immune cells.
This enables the identification of optimal conditions for co-culturing various immune cells, facilitating the selection of an appropriate platform for drug testing.
The co-culture drug evaluation solution with macrophages, T cells, and cancer organoids allows for the simultaneous observation of the phagocytic activity of macrophages and the cytotoxic ability of T cells. Co-culturing with both M1 and M2 macrophages is possible, mimicking the role of macrophages observed in actual cancer tissues.
By utilizing patient-derived tumor organoids and T cells, macrophages, this is a new evaluation solution with mechanisms involving two or more immune cells.
Generally, M1 macrophages are known to be associated with tumor growth suppression and M2 macrophages are known to be associated with tumor growth promotion.
To perform different functions, they have different immune markers, metabolic characteristics and gene profiles.
In vivo, proper balance of differentiation into M1 and M2 macrophages is required to elicit an immune response against tumors.
For the development of a new drug evaluation solution, we conducted efficacy evaluations of drugs and antibodies on co-cultures of M1 macrophages, M2 macrophages and tumor organoids.
After conducting the efficacy evaluation, growth rates and mortality were measured to establish optimal co-culture conditions
To enable efficient testing, we have developed an evaluation solution utilizing macrophages derived from pluripotent stem cells.
Ultimately, we have created an optimal co-culture model with macrophages derived from pluripotent stem cells, T cells and tumor organoids. By testing various drug conditions on this model, we confirmed high drug response rates.
We have selected these rates as evaluation criteria for immuno-oncology drugs.
I suggest challenging more advanced research through this new evaluation solution involving multiple immune cells.
It could be a more effective strategy for your research.
A drug evaluation solution co-culturing Treg, T cells, and cancer organoids allows for the assessment of drugs that can regulate Treg.
This provides a drug evaluation solution capable of evaluating drugs that can modulate Treg.
A drug evaluation solution co-culturing Treg, T cells, and cancer organoids allows for the assessment of drugs that can regulate Treg. This provides a drug evaluation solution capable of evaluating drugs that can modulate Treg.
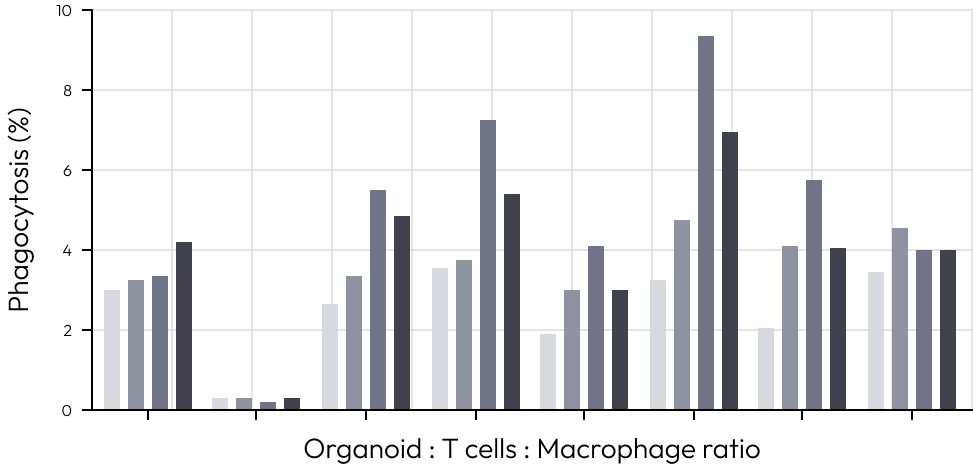
We aimed to develop tumor organoids with a tumor microenvironment and use them as a solution for drug evaluation.
For this, we created a system where tumor organoids, cytotoxic T cells, and regulatory T cells were co-cultured at specific ratios.
We activated T cells from PBMCs, then separately cultured and counted cytotoxic T cells and regulatory T cells.
Finally, after establishing the co-culture system at designated ratios, we measured the efficacy of immuno-oncology drugs.
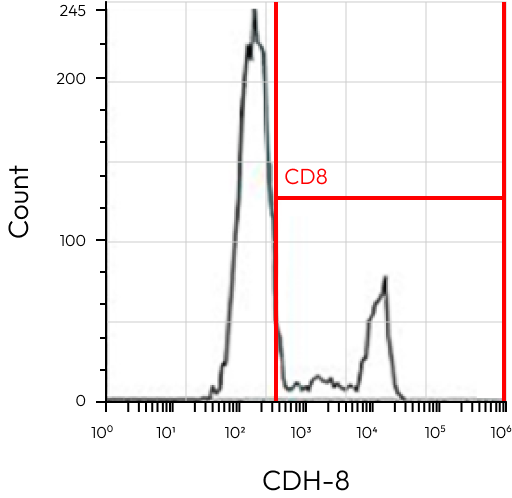
R1 / T-cell
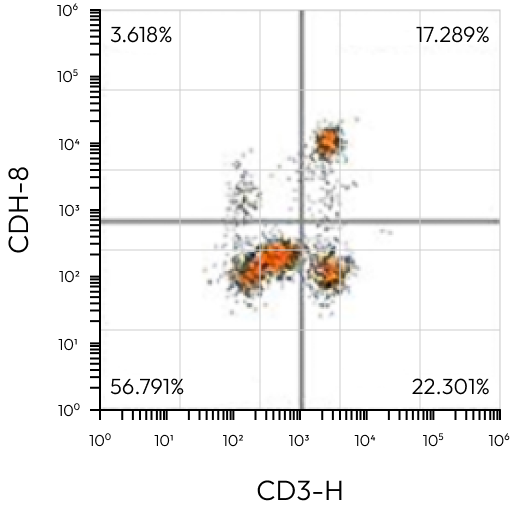
R1 / T-cell
H&E | CK7 | |
|---|---|---|
CD8+ | 47,600 | – |
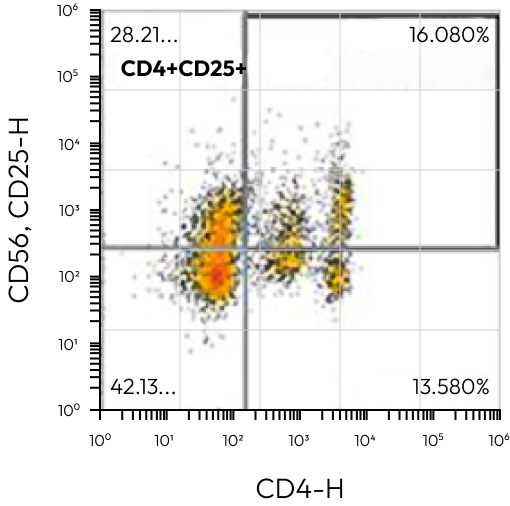
All Events / Treg
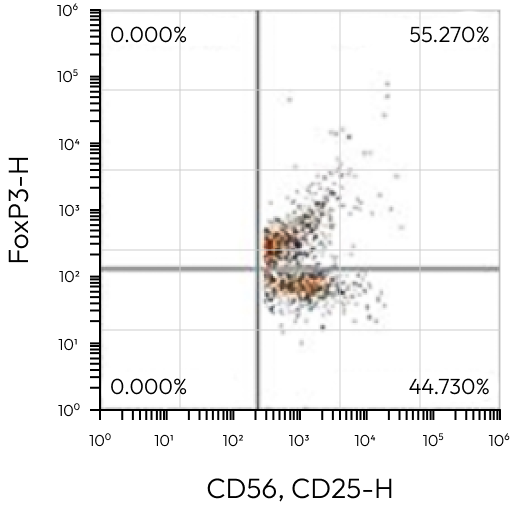
CD4+CD5+ / Treg
H&E | CK7 | |
|---|---|---|
CD8+CD25+FoxP3 | 7,500 | – |
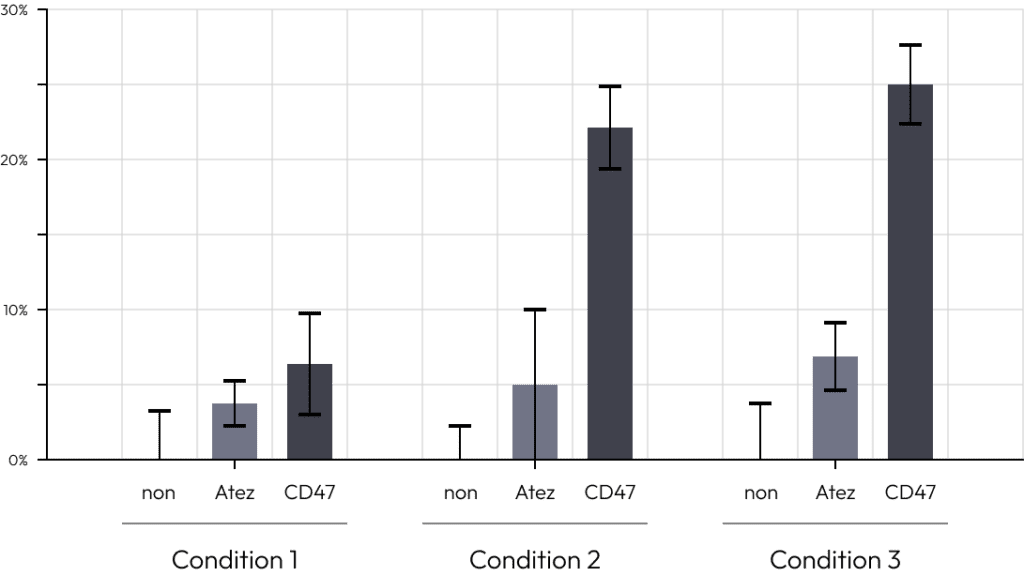
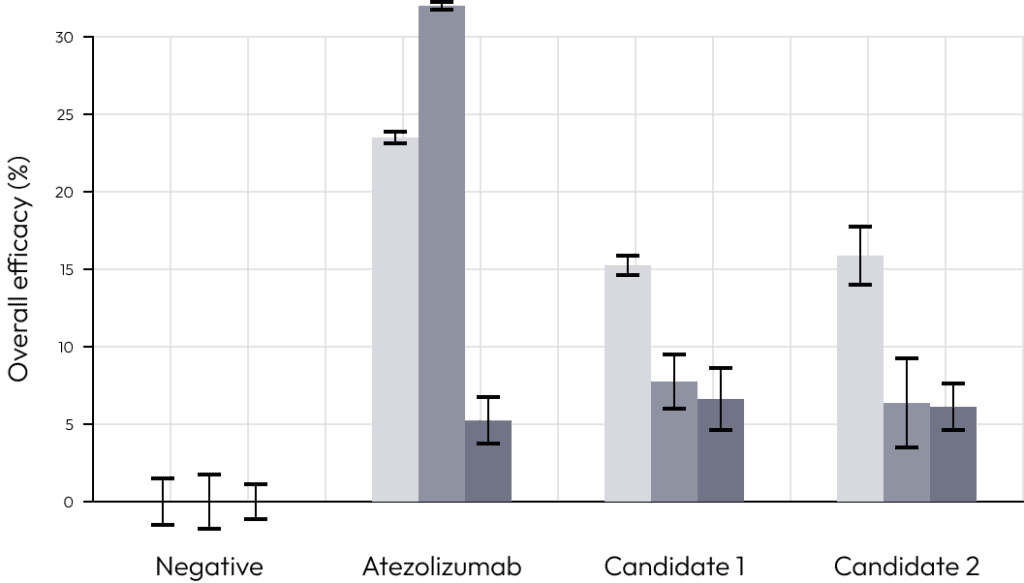
We compared the commonly used immuno-oncology drug atezolizumab with candidate.
When T cells alone or cytotoxic T cells and regulatory T cells were added, we examined the effect of each immuno-oncology drug.
Only in the case of co-culture with regulatory T cells, the efficacy of the drug decreased rapidly.
This is a result of regulatory T cells inducing T cell suppression during co-culture.
These results prove that our solution can most similarly simulate the tumor microenvironment consisting of tumor organoids, cytotoxic T cells and regulatory T cells.
Screen and accurately evaluate anti-cancer drugs targeting regulatory T cells using our validated solution that closely resembles the in vivo tumor microenvironment.
Tumor-infiltrating lymphocytes (TILs) are immune cells in cancer tissues with the potential to either eliminate or aid in cancer growth.
Immunotherapeutics can reactivate exhausted TILs, allowing them to attack cancer cells.
Direct extraction and cultivation of TILs provide an evaluation solution for immunotherapeutics, and the extraction yield can predict their responsiveness, considering potential absence due to extraction yield or unique cancer characteristics.
Tumor-infiltrating lymphocytes (TILs) are immune cells in cancer tissues with the potential to either eliminate or aid in cancer growth.
Immunotherapeutics can reactivate exhausted TILs, allowing them to attack cancer cells.
Direct extraction and cultivation of TILs provide an evaluation solution for immunotherapeutics, and the extraction yield can predict their responsiveness, considering potential absence due to extraction yield or unique cancer characteristics.
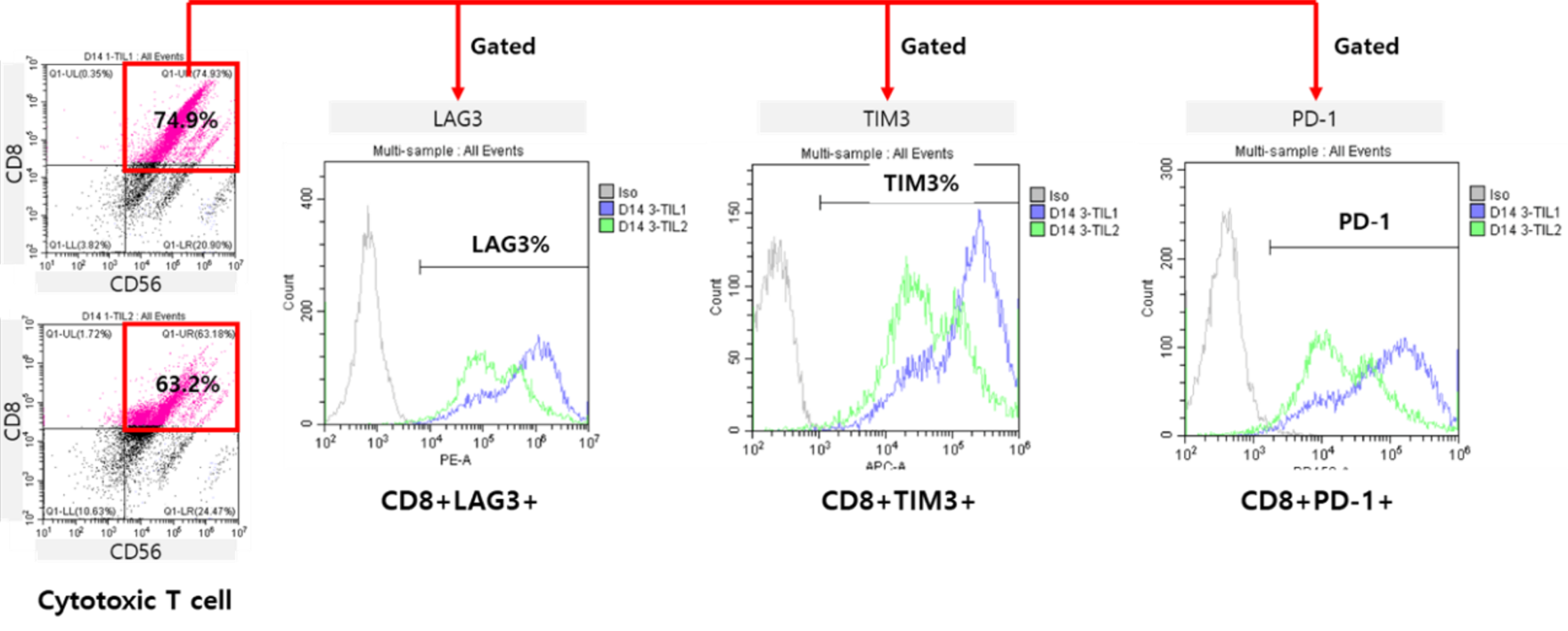
Tumor-infiltrating lymphocytes (TIL) refer to lymphocytes that infiltrate tumors either intrinsically or extrinsically to eliminate tumors or help tumor growth.
Some TIL have antitumor reactivity that can eliminate tumor cells, but they often exist in an inhibited state.
Our company has developed conditions to extract and culture TIL from patients’ tumors.
During TIL culture, the number of cytotoxic T cells increased, and the expression levels of various immune checkpoint molecules allowed us to check the status of TIL.
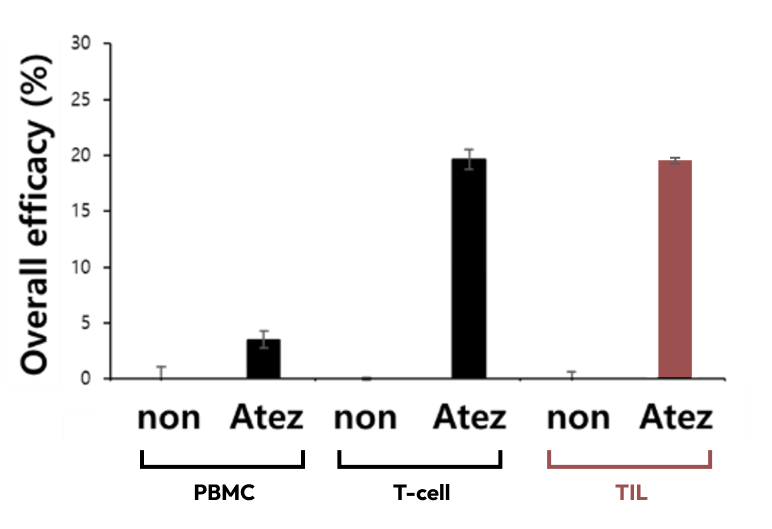
Overall efficacy (Death cells)
When evaluating the efficacy of atezolizumab in co-culture conditions with tumor organoids and tumor-infiltrating lymphocytes or tumor organoids and T cells derived from PBMCs, higher tumor organoid killing was observed in conditions without co-culture with tumor-infiltrating lymphocytes or T cells.
The method of evaluating efficacy by treating a specific mixture of tumor organoids and tumor-infiltrating lymphocytes with anticancer drugs is still at the research level globally, and our company uniquely holds patents and commercialization services related to this.
Make your research shine brighter through the only service of its kind in the world.
Evaluation of the efficacy of an immunotherapeutic agent targeting cytotoxic T cells is possible by co-culturing specific organoids with Primed Cytotoxic T cells, allowing for the selective elimination of particular organoids.
This provides a solution for assessing the effectiveness of immunotherapeutic agents targeting cytotoxic T cells in co-culture with cancer organoids.
Evaluation of the efficacy of an immunotherapeutic agent targeting cytotoxic T cells is possible by co-culturing specific organoids with Primed
Cytotoxic T cells, allowing for the selective elimination of particular organoids.
This provides a solution for assessing the effectiveness of immunotherapeutic agents targeting cytotoxic T cells in co-culture with cancer organoids.
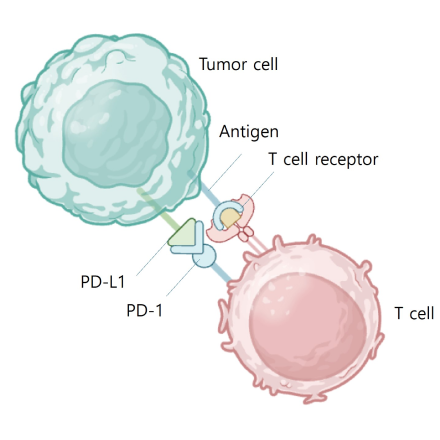
PD L1 binds to PD 1 and inhibits T cell kiling of tumor cell
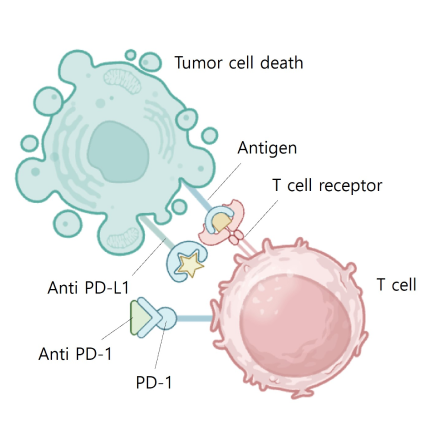
Blocking PD L1 or PD 1 allows T cell kiling of tumor cell
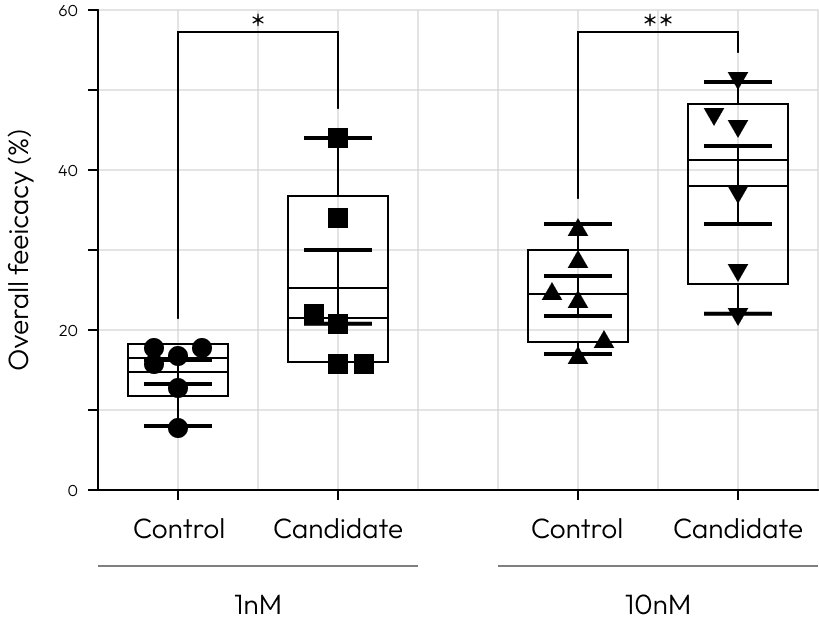
High Responder to control
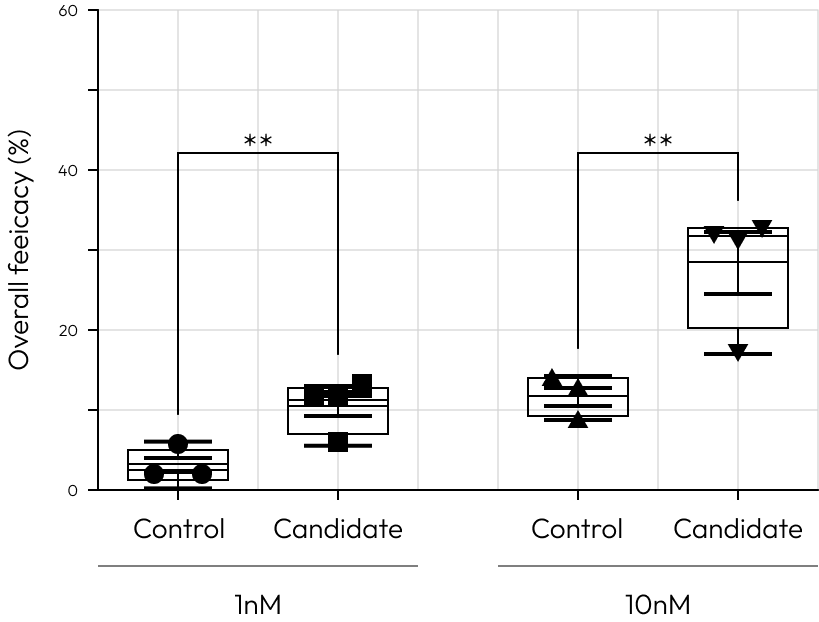
Low Responder to control
Our cutting-edge platform brings approach in drug development, empowering clinicians and researchers to transcend beyond the binary classification of responders versus non-responders.
It facilitates a deeper examination of patient-specific responses to approved control drugs at various concentrations.
This technology illuminates the intricate interplay between genetic, regional, and environmental factors and their impact on drug efficacy, providing a path to address the pressing unmet needs in before clinical trail.
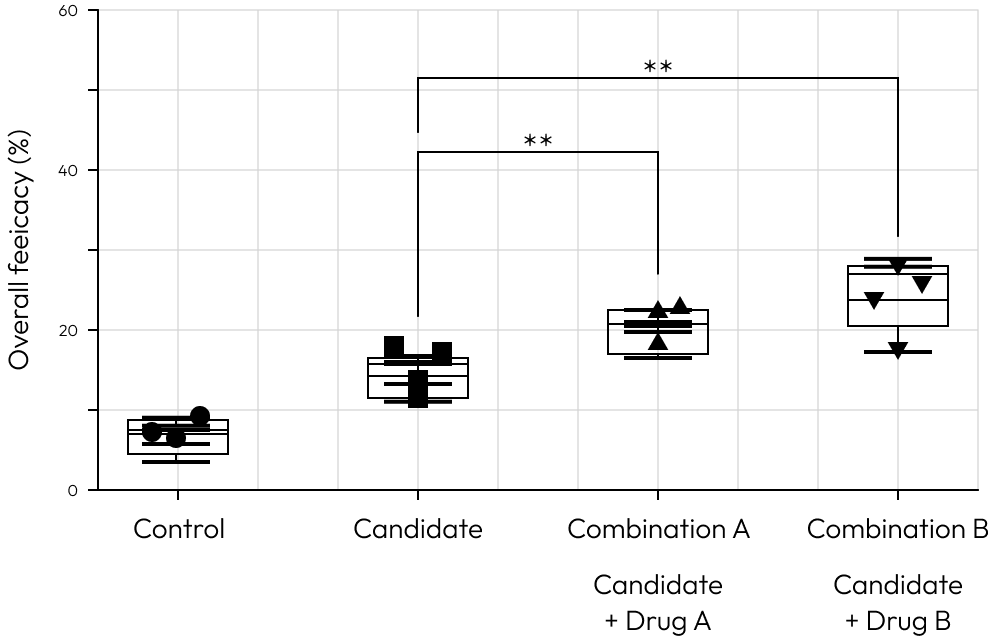
Combination
This example showcases the potential utility of our platform in identifying optimal partners for combination therapy with Immune Checkpoint Inhibitors (ICIs).
His suggests that Combination B could be a more effective strategy in enhancing the efficacy of ICIs.
This platform is designed to facilitate such discoveries, offering a clear pathway to improve clinical outcomes through strategic drug pairing.