The oncology drug efficacy evaluation platform is based on patient-derived organoids(PDOs). This platform allows for the assessment of the efficacy of oncology agents and immunotherapeutics by utilizing a co-culture system with immune cells from the tumor microenvironment.
Our biobanking system contains over 16 various cancer types, with 500 cases, all across 3 different ethnicities.
Quality control and assurance methodologies applied across all cancer types – Whole exome sequencing, drug sensitivity testing with clinical patient information.
Costs start at €1.000 per case, with full results within 2 weeks.
Co-culturing of immune cells with CAFs allows for the analysis of complex cell interactions within the tumor microenvironment, allowing for precise drug screening and testing.
Our biobanking system contains over 16 various cancer types, with 500 cases, all across 3 different ethnicities.
Quality control and assurance methodologies applied across all cancer types – Whole exome sequencing, drug sensitivity testing with clinical patient information.
Costs start at €1.000 per case, with full results within 2 weeks.
Co-culturing of immune cells with CAFs allows for the analysis of complex cell interactions within the tumor microenvironment, allowing for precise drug screening and testing.
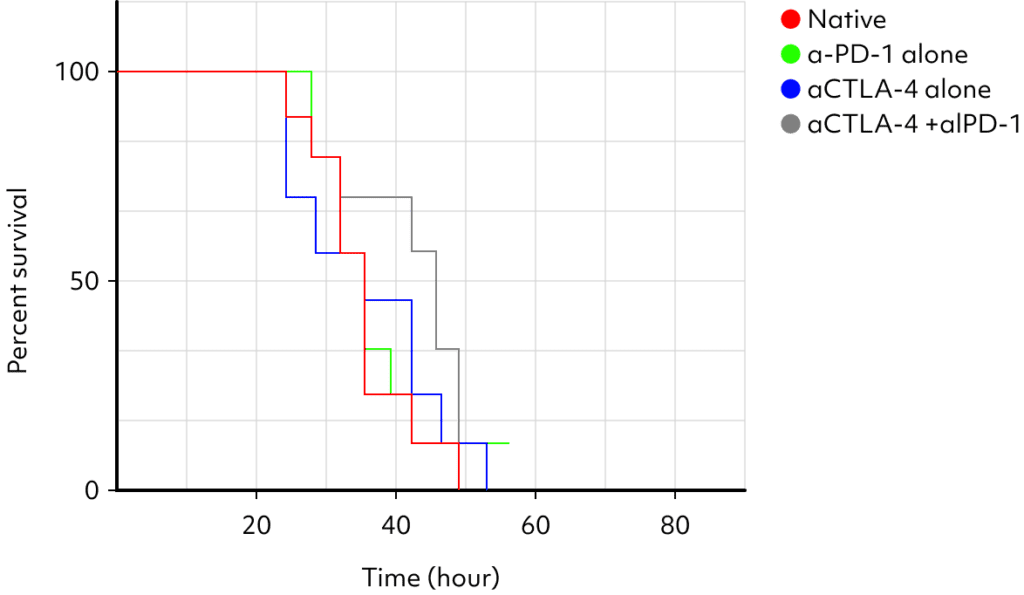
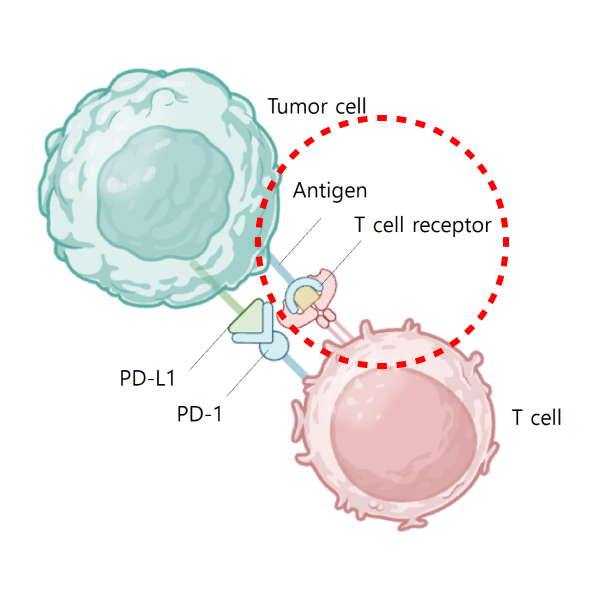
Although the drug has proven efficacy in humans, its effectiveness has not been observed
Optimize with Confidence: Leading the Way in Lead Selection Using Advanced Organoid Systems.
This systems are designed to guide you in selecting the most promising drug candidates for clinical trials.
By simulating human biological conditions closely, we help identify leads with the highest potential for success.
This approach minimizes risks and maximizes the likelihood of clinical trial success, ensuring that you advance only the best candidates into critical stages of drug development.
Unlocking Tumor Killing Potential with Precision T Cell Engagement.
Our evaluation process excels in identifying the most promising therapeutic agents, setting the stage for treatments tailored to optimal response conditions.
This approach uncovers potential synergistic effects, enhancing the overall efficacy and impact of new combination.
Elevate Immune Checkpoint Inhibitor Success: Tailored Efficacy Comparisons Using Cutting-Edge Organoid Models.
This systems are designed to guide you in selecting the most promising drug candidates for clinical trials.
By simulating human biological conditions closely, we help identify leads with the highest potential for success.
This approach minimizes risks and maximizes the likelihood of clinical trial success, ensuring that you advance only the best candidates into critical stages of drug development
Revolutionizing Cancer Discovery: Unveiling Hidden Targets with Advanced Organoid-Immune Cell Models.
Step into the future of cancer treatment with our pioneering organoid models.
By analyzing a wide range of tumor organoids with distinct mutations, we provide unparalleled insights into the most effective biomarkers and tumor targets for your therapeutic compounds.
Our approach not only accelerates the discovery of novel targets but also enhances the precision and effectiveness of your drug development process.

Proven Efficacy in Complex Tumor Microenvironments.
Validate the efficacy of bispecific antibodies.
Explore the potential of these advanced therapies in complex tumor microenvironments, and consider the intriguing possibilities of trispecific antibodies for even greater therapeutic impact.
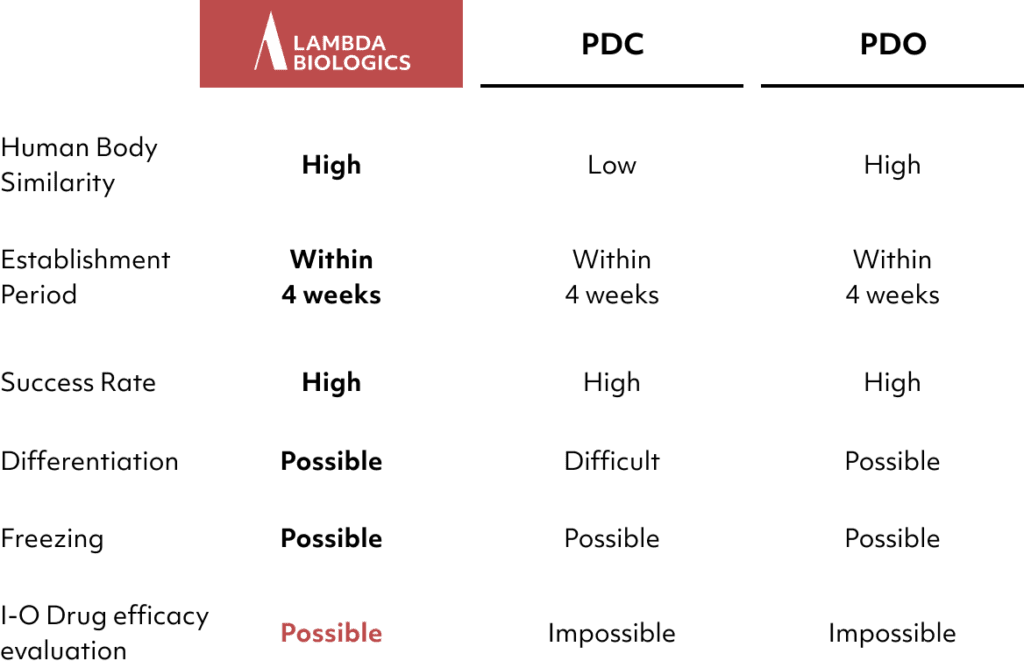

Provides customized solutions based on the ability to produce various organoids and in vivo mimicry technology, addressing diverse unmet needs and offering multiple patented products.



Lambda currently possesses PDO models for three types of cancer, enabling the provision of organoid services.
Additionally, preparations are underway to offer services for 13 different types of cancer. Lambda supports research in anti-cancer agents and tumor studies.
Lambda currently possesses PDO models for three types of cancer, enabling the provision of organoid services.
Additionally, preparations are underway to offer services for 13 different types of cancer. Lambda supports research in anti-cancer agents and tumor studies.
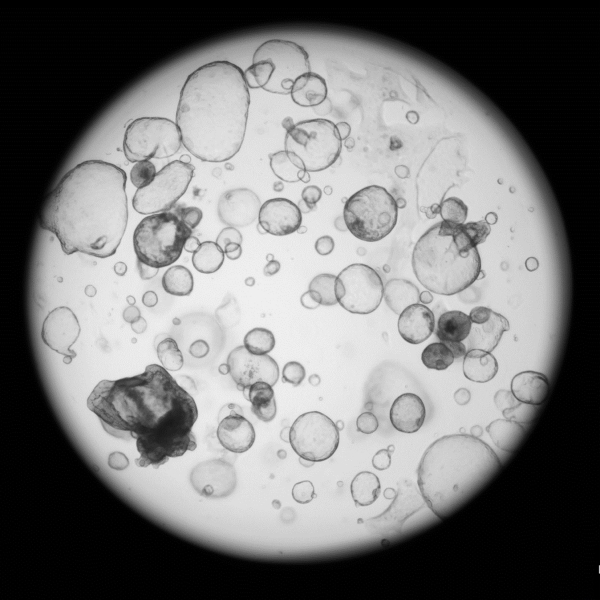
Preparing
Gastric
cancer organoid

Preparing
Cholangiocarcinoma
organoid
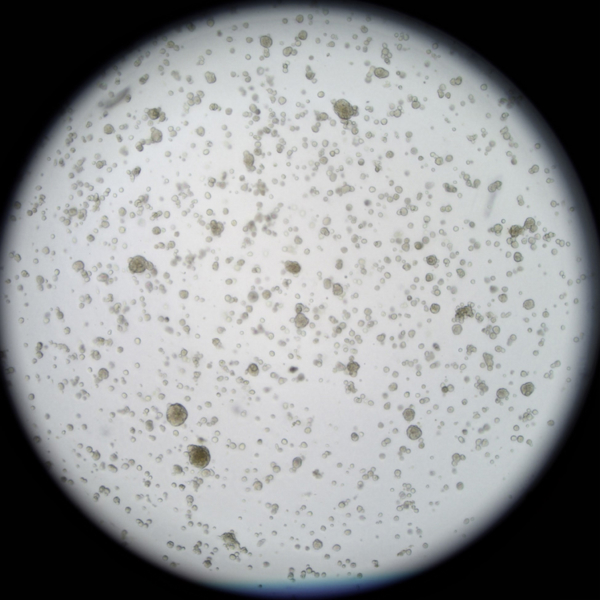
Preparing
Breast
cancer organoid
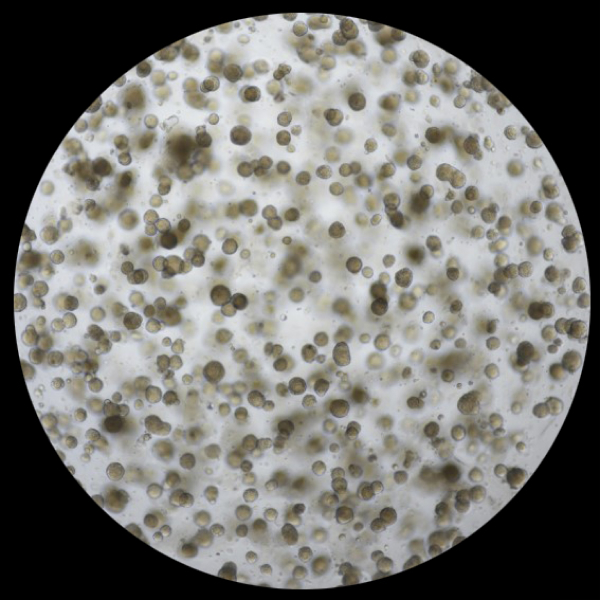
Preparing
Esophageal cancer organoid
Preparing
Hepatocarcinoma cancer organoid
Preparing
Melanoma organoid
Preparing
Bladder cancer organoid
Preparing
Prostate cancer organoid
Preparing
Renal cell carcinoma organoid
Preparing
Endometrial cancer organoid
Preparing
Head & Neck cancer organoid
Preparing
Ovarian cancer organoid
Preparing
Cervical cancer organoid
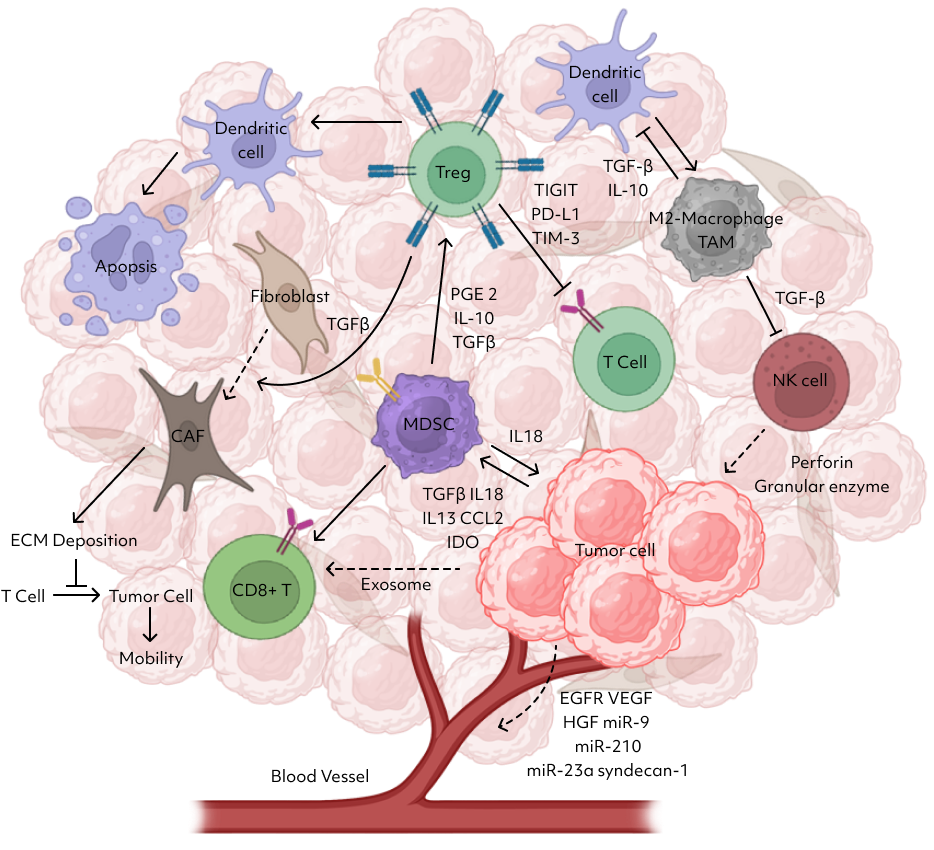
Lambda has established a system that incorporates five elements constituting the tumor microenvironment and enables co-culturing with tumors.
This co-culture system demonstrates higher resemblance and mimicry to actual tumor tissues compared to PDO models, providing a more accurate reflection of drug responses similar to those observed in real tumors.
Lambda has established a system that incorporates five elements constituting the tumor microenvironment and enables co-culturing with tumors.
This co-culture system demonstrates higher resemblance and mimicry to actual tumor tissues compared to PDO models, providing a more accurate reflection of drug responses similar to those observed in real tumors.
Establishing a process for isolating cytotoxic T cells that can directly attack cancer cells, creating an MHC-TCR interaction environment, and utilizing MHC I/II blockers to achieve an immune microenvironment in vivo.
Since obtaining blood from cancer patients is limited, the process is refined by co-culturing peripheral blood mononuclear cells (PBMC) derived from healthy individuals with cancer organoids from cancer patients. This allows for the generation of cytotoxic T cells.
A co-culturing platform of macrophages and organoids demonstrates varying organoid cytotoxic effects depending on the ratio of M1 macrophages to M2 macrophages. Optimal cell death effects are established by co-culturing different ratios of functionally diverse immune cells. This enables the identification of optimal conditions for co-culturing various immune cells, facilitating the selection of an appropriate platform for drug testing.
Regulatory T cells (Treg)” play a crucial role in maintaining immune homeostasis and tolerance by suppressing immune responses.
These lymphocytes inhibit T cell proliferation and cytokine production, thus preventing autoimmune reactions.
Our method involves evaluating the efficacy of an anticancer agent by treating a mixture of tumor organoids, cytotoxic T cells (CD8+ T cells), and regulatory T cells (Tregs) with the anticancer drug or drug candidate.
Tumor-Infiltrating Lymphocytes (TILs) are predominantly found in the microenvironment of tumors, including various types of lymphocytes that have infiltrated in and around tumor tissues. Among them, CD8+ cytotoxic T cells and CD4+ helper T cells are major components. Our approach involves isolating and expanding TILs from a patient’s tumor tissue, then evaluating the efficacy of anticancer agents in a tumor microenvironment containing cancer organoids and TILs.
Cancer-associated fibroblasts (CAFs) play a crucial role in the microenvironment of cancer.
These cells are present within and/or around cancer lesions, and in cancer types where CAFs are concentrated, there is often a low response to anticancer drugs.
Additionally, the maintenance of a CAF-centric cancer microenvironment can lead to a higher likelihood of recurrence, even if there is a tumor destruction effect.
We have established a method for isolating CAFs, defined co-culture conditions for CAFs and organoids (including cell ratios and culture medium composition), and validated the ability to evaluate drug responses to immunotherapeutic agents.
NK cells’ presence and activity in thetumor predict patient responses to treatments, linking drug efficacy to NK cellrecruitment and function.
NK cells actively surveil and eliminate cancer cellsearly on, and evaluating drug impact on NK cell-mediated immunosurveillanceassesses potential in preventing tumor development.
Anti-cancer drugs influencethe immune microenvironment, highlighting the importance of understanding NKcell and immune milieu interplay for overall drug efficacy assessment.
MDSCs, or myeloid-derived suppressor cells, create an immunosuppressive environment within tumors by inhibiting the activity of immune cells like Tcells and NK cells.
This hampers the overall anti-cancer immune response.
Moreover, MDSCs contribute to angiogenesis and metastasis, complicating theeffectiveness of drugs targeting these processes.
The dual role of MDSCs as immunosuppressors and promoters of tumor-related processes poses challenges fortherapies aiming to impede these crucial aspects of cancer progression.