The U.S. Food and Drug Administration issued a draft guidance (December 2, 2025) describing when six-month non-human primate (NHP) toxicity studies may be eliminated or reduced for certain monoclonal antibodies. The guidance frames a risk-based approach that incorporates human-relevant data streams – including computational toxicology, organoid systems, and clinical safety data – as part of regulatory decision making.
A typical monoclonal antibody program can involve more than 100 NHPs (often macaques), with per-animal costs cited at roughly $50,000 – yet many products that pass animal toxicity testing later fail in the clinic for human-specific safety or efficacy reasons. The FDA highlights these gaps as part of its rationale for a more targeted, evidence-based testing strategy.
The FDA’s draft guidance outlines a knowledge-based risk assessment that evaluates product characteristics (target biology, distribution, binding kinetics) and the totality of available data to decide whether long-term primate studies are necessary. In some cases, the agency indicates shorter animal studies or reliance on alternative data may suffice. Industry coverage notes the FDA specifically suggested that some new monospecific antibodies may not require six-month primate studies and, where appropriate, sponsors could use shorter (e.g., three-month) animal studies plus existing class data – or in some situations waive even three-month studies.
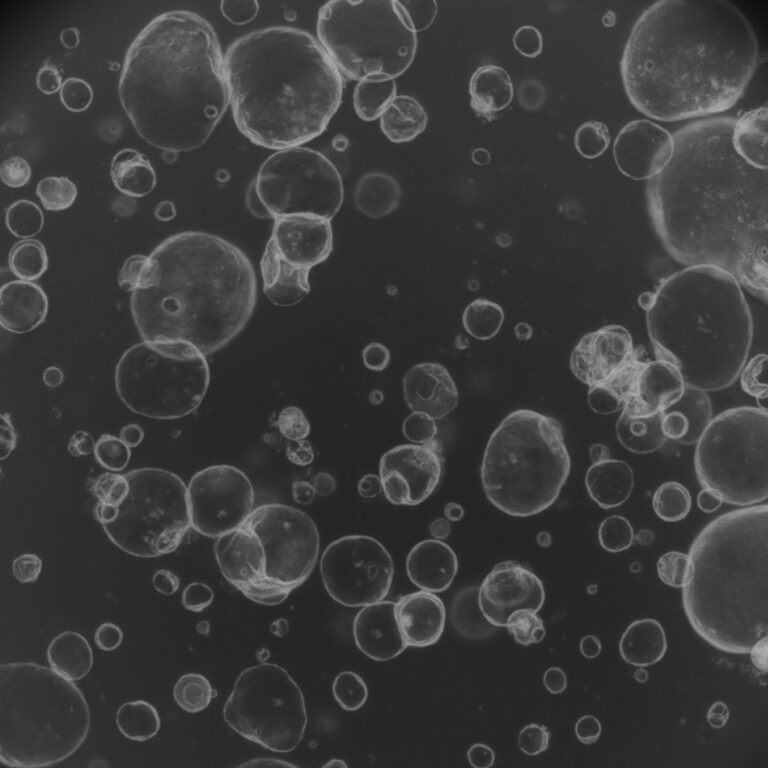
The guidance explicitly promotes integrating NAMs such as in silico predictive models, human organoid and other advanced in-vitro systems, and aggregated clinical/post-marketing safety data into nonclinical packages. The FDA also signals continued collaboration with federal partners (NIH, ICCVAM) and international bodies to validate and expand NAMs. Separately, reporting from other outlets highlights national moves – including federal attention to reducing some primate research and NIH investments in organoid modeling centers – that underscore broader momentum toward animal-free methods.
When scientifically justified, reducing or eliminating long-term NHP studies can shorten IND timelines, cut program costs, and shift focus toward human-relevant mechanistic data that may better predict clinical outcomes – while also advancing 3Rs ethical goals. The FDA’s draft guidance marks a meaningful regulatory step toward modernizing toxicology around human biology and integrated risk assessment.
Read more:
- FDA Releases Draft Guidance on Reducing Testing on Non-Human Primates for Monoclonal Antibodies
- FDA issues draft guidance to reduce primate testing for certain antibodies
- The U.S. Centers for Disease Control and Prevention plans to end monkey testing by the end of the year.