Establishing a process for isolating cytotoxic T cells that can directly attack cancer cells, creating an MHC-TCR interaction environment, and utilizing MHC I/II blockers to achieve an immune microenvironment in vivo.
Since obtaining blood from cancer patients is limited, the process is refined by co-culturing peripheral blood mononuclear cells (PBMC) derived from healthy individuals with cancer organoids from cancer patients. This allows for the generation of cytotoxic T cells.

Cytotoxic T cell
Colorectal cancer organoids accurately replicate the complexities of patient tumors, including heterogeneity,
genetic traits, and tissue structure.
These organoids serve as a versatile platform for drug testing, allowing the evaluation of drug responses and sensitivity.
Additionally, they facilitate personalized disease modeling using patient-specific samples, contributing to biomarker identification for prognosis and treatment response.
NSCLC organoids, reflecting the heterogeneity and genetic features of patient tumors, provide a versatile platform for in-depth cancer research.
These organoids faithfully mimic the tissue architecture of NSCLC, facilitating the study of tumor dynamics, invasion patterns, and drug responses.
Their patient-specific modeling capability allows personalized exploration of treatment outcomes.
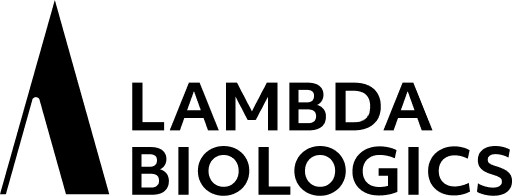
Evaluation of the efficacy of an immunotherapeutic agent targeting cytotoxic T cells is possible by co-culturing specific organoids with Primed
Cytotoxic T cells, allowing for the selective elimination of particular organoids.
This provides a solution for assessing the effectiveness of immunotherapeutic agents targeting cytotoxic T cells in co-culture with cancer organoids.
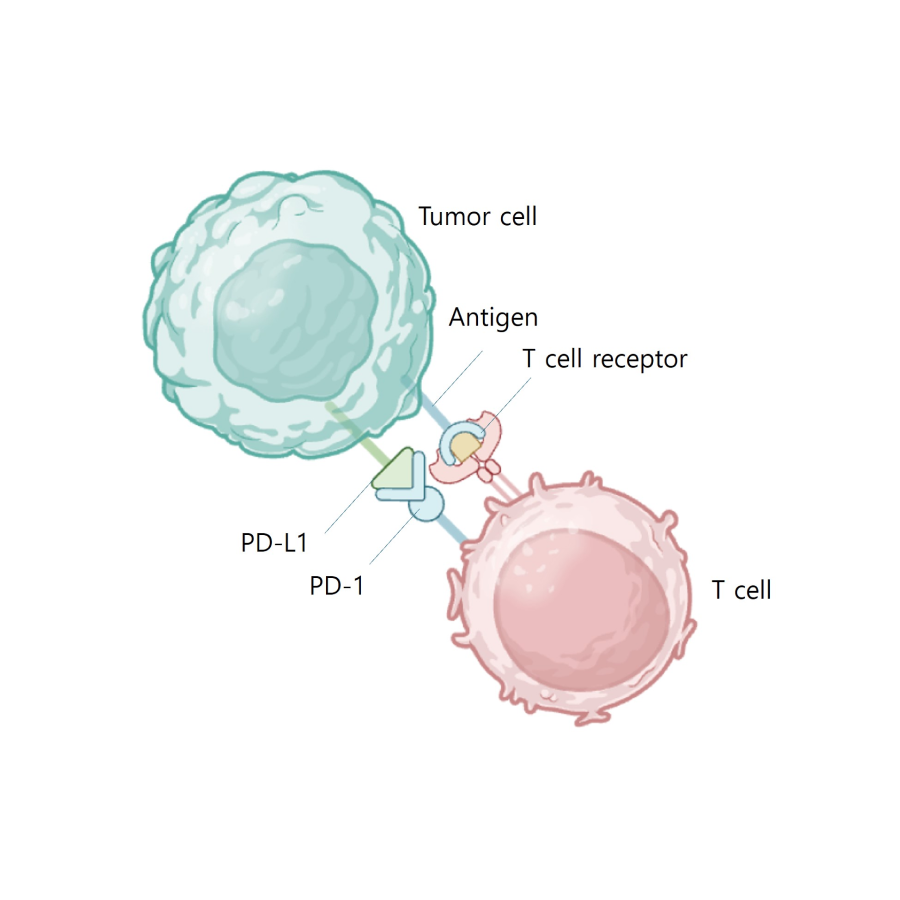
PD L1 binds to PD 1 and inhibits
T cell kiling of tumor cell
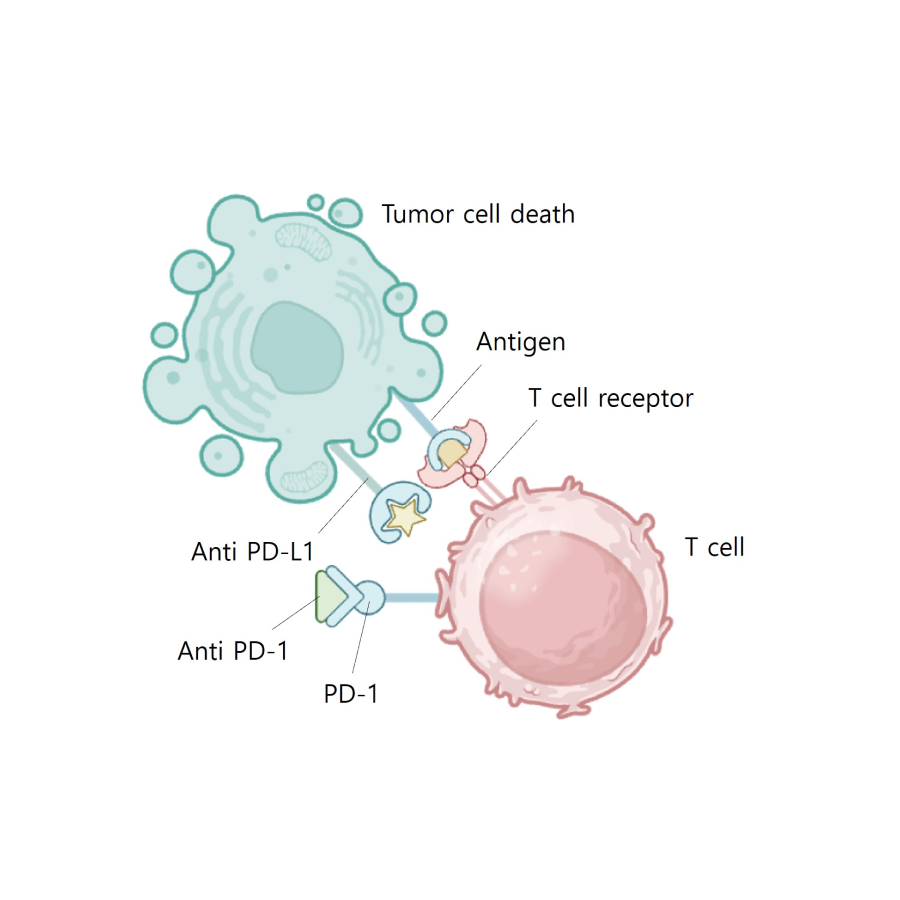
Blocking PD L1 or PD 1 allows
T cell kiling of tumor cell
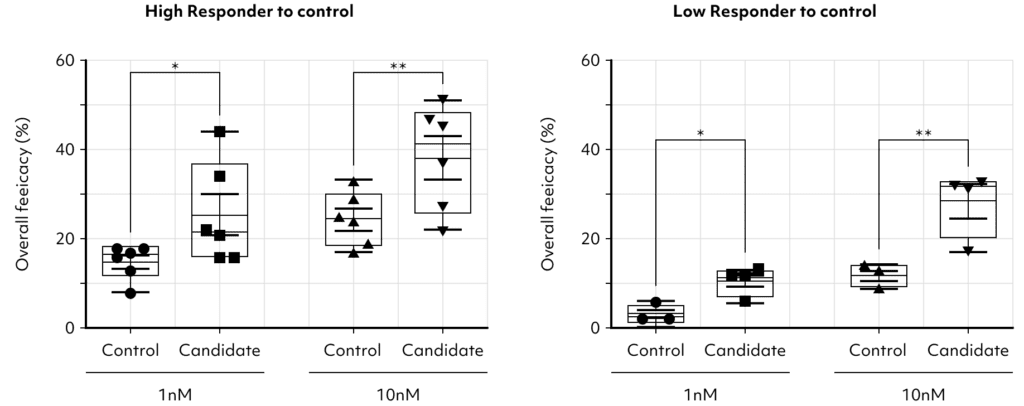
Our cutting-edge platform brings approach in drug development, empowering clinicians and
researchers to transcend beyond the binary classification of responders versus non-responders.
It facilitates a deeper examination of patient-specific responses to approved control drugs at various concentrations.
This technology illuminates the intricate interplay between genetic, regional, and environmental factors and
their impact on drug efficacy, providing a path to address the pressing unmet needs in before clinical trail.
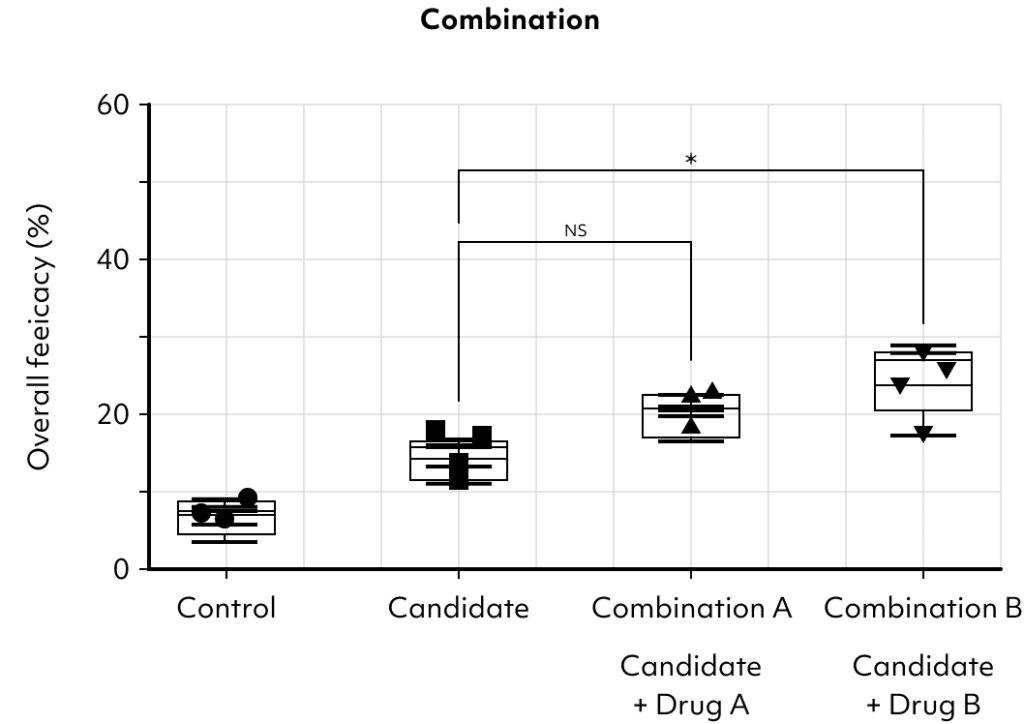
This example showcases the potential utility of our platform in identifying optimal partners for combination therapy with Immune Checkpoint Inhibitors (ICIs).
his suggests that Combination B could be a more effective strategy in enhancing the efficacy of ICIs.
This platform is designed to facilitate such discoveries, offering a clear pathway to improve clinical outcomes through strategic drug pairing.