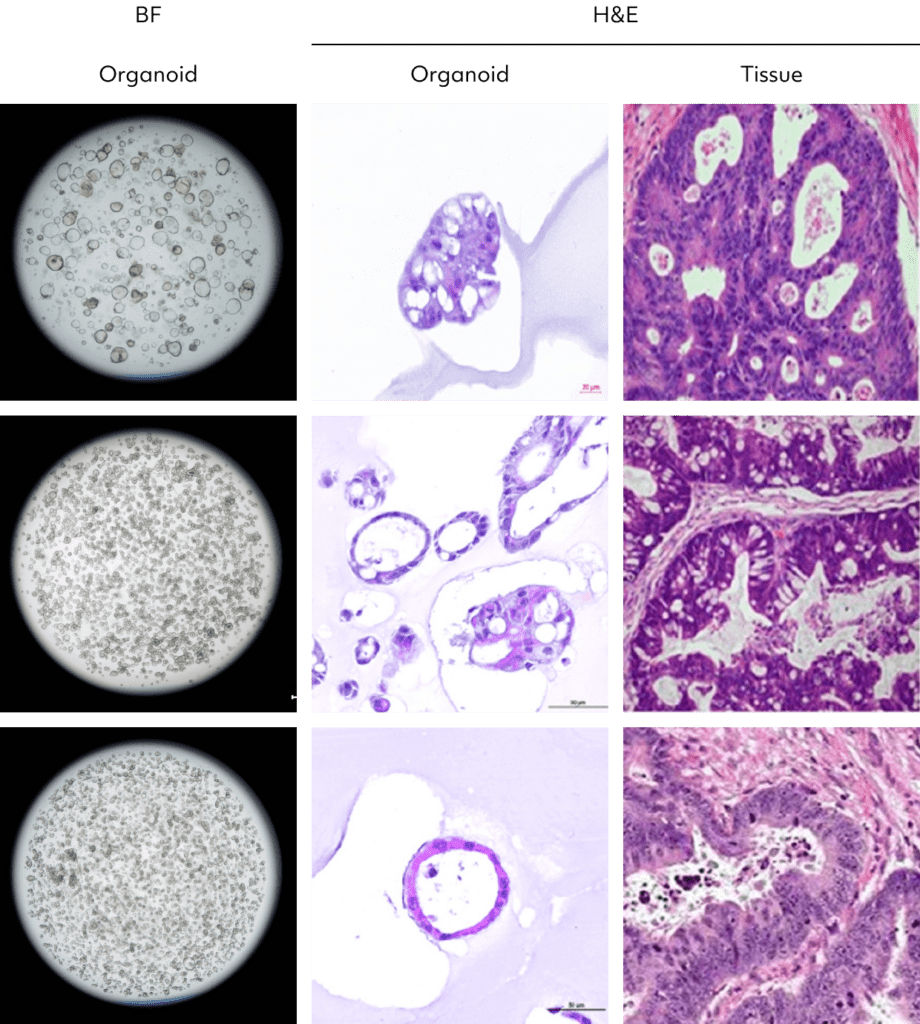
Colorectal cancer organoids accurately replicate the complexities of patient tumors, including heterogeneity,
genetic traits, and tissue structure.
These organoids serve as a versatile platform for drug testing, allowing the evaluation of drug responses and sensitivity.
Additionally, they facilitate personalized disease modeling using patient-specific samples, contributing to biomarker identification for prognosis and treatment response.
The incorporation of high-throughput drug screening and consideration of the tumor microenvironment further enhances the potential of colorectal cancer organoids in advancing our comprehension of the disease and tailoring precision medicine strategies.

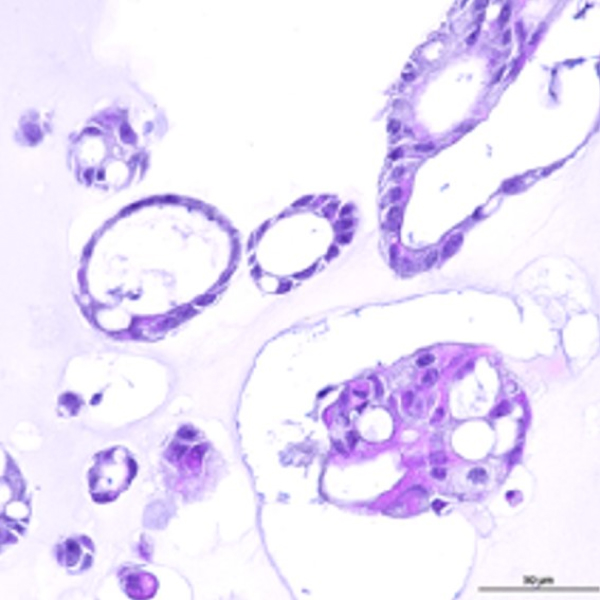

Colorectal
cancer organoid
Establishing a process for isolating cytotoxic T cells that can directly attack cancer cells, creating an MHC-TCR interaction environment, and utilizing MHC I/II blockers to achieve an immune microenvironment in vivo.
Since obtaining blood from cancer patients is limited, the process is refined by co-culturing peripheral blood mononuclear cells (PBMC) derived from healthy individuals with cancer organoids from cancer patients. This allows for the generation of cytotoxic T cells.
Regulatory T cells (Treg)” play a crucial role in maintaining immune homeostasis and tolerance by suppressing immune responses.
These lymphocytes inhibit T cell proliferation and cytokine production, thus preventing autoimmune reactions.
Our method involves evaluating the efficacy of an anticancer agent by treating a mixture of tumor organoids, cytotoxic T cells (CD8+ T cells), and regulatory T cells (Tregs) with the anticancer drug or drug candidate.
A co-culturing platform of macrophages and organoids demonstrates varying organoid cytotoxic effects depending on the ratio of M1 macrophages to M2 macrophages. Optimal cell death effects are established by co-culturing different ratios of functionally diverse immune cells. This enables the identification of optimal conditions for co-culturing various immune cells, facilitating the selection of an appropriate platform for drug testing.
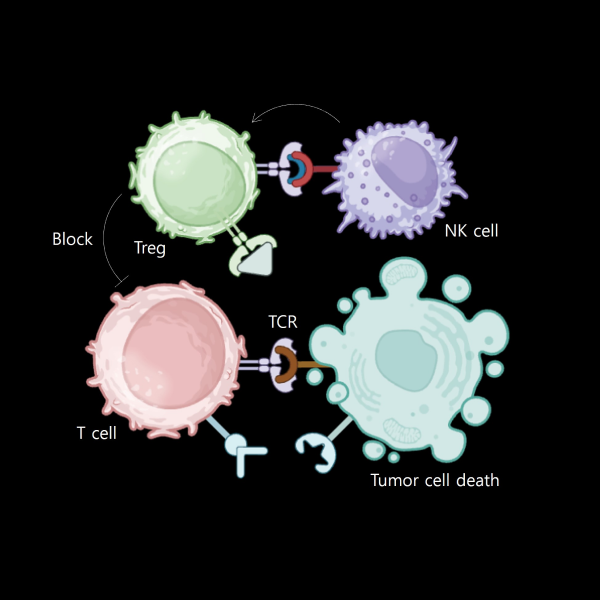
Preparing
NK cell
NK cells’ presence and activity in the tumor predict patient responses to treatments, linking drug efficacy to NK cell recruitment and function. NK cells actively surveil and eliminate cancer cells early on, and evaluating drug impact on NK cell-mediated immunosurveillance assesses potential in preventing tumor development. Anti-cancer drugs influence the immune microenvironment, highlighting the importance of understanding NK cell and immune milieu interplay for overall drug efficacy assessment.